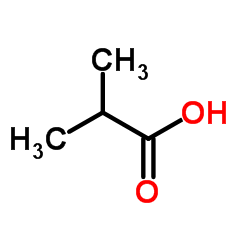
303-45-7
| Name | gossypol |
|---|---|
| Synonyms |
MFCD00017352
1,1',6,6',7,7'-Hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl[2,2'-binaphthalene]-8,8'-dicarboxaldehyde 1,1',6,6',7,7'-Hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl-2,2'-binaphthalene-8,8'-dicarbaldehyde 2,2'-BI[8-FORMYL-1,6,7-TRIHYDROXY-5-ISOPROPYL-3-METHYLNAPHTHALENE] 2,2'-Bis[1,6,7-trihydroxy-3-methyl-5-isopropyl-8-aldehydonaphthalene] 1,1',6,6',7,7'-hexahydroxy-3,3'-dimethyl-5,5'-di(propan-2-yl)-2,2'-binaphthalene-8,8'-dicarbaldehyde Gossypol gossypol from cotton seeds (rac)-2,2'-bis(8-formyl-1,6,7-trihydroxy-5-isopropyl-3-methylnaphthalene) 2,2'-bis[8-formyl-1,6,7-trihydroxy-5-isopropyl-3-methylnaphthalene] (-)-1,1',6,6',7,7'-Hexahydroxy-3,3'-dimethyl-5,5'-bis(1-methylethyl)[2,2'-binaphthalene]-8,8'-dicarboxaldehyde 2,2'-BIS[8-FORMYL-1,6,7-TRIHYDROXY-5-ISOPROPYL-3-METHYLNAPTHALENE] 1,1',6,6',7,7'-hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl-(2,2'-binaphthalene)-8,8'-dicarboxaldehyde 1,1',6,6',7,7'-Hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl-2,2'-binaphthalen-8,8'-dicarbaldehyd Thespesin [2,2'-Binaphthalene]-8,8'-dicarboxaldehyde, 1,1',6,6',7,7'-hexahydroxy-3,3'-dimethyl-5,5'-bis(1-methylethyl)- 1,1',6,6',7,7'-hexahydroxy-3,3'-dimethyl-5,5'-bis(1-methylethyl)-2,2'-binaphthalene-8,8'-dicarbaldehyde roduct Name (+/-)-gossypol 1,1',6,6',7,7'-Hexahydroxy-3,3'-dimethyl-5,5'-bis(1-methylethyl)[2,2'-binaphthalene]-8,8'-dicarboxaldehyde |
| Description | Gossypol, a natural product isolated from cottonseeds and roots, binds to Bcl-xL protein and Bcl-2 protein with Kis of 0.5-0.6 μM and 0.2-0.3 mM, respectively. |
|---|---|
| Related Catalog | |
| Target |
Bcl-xL:0.5-0.6 μM (Ki) Bcl-2:0.2-0.3 mM (Ki) |
| In Vitro | Gossypol, a natural product isolated from cottonseeds and roots that has been studied as an anticancer agent. The racemic form of Gossypol [(±)-Gossypol] is tested in several clinical trials and is well tolerated. The racemic form Gossypol ((±)-Gossypol) binds to Bcl-xL protein with a Ki of 0.5 to 0.6 μM. (±)-Gossypol also potently binds to Bcl-2 protein with a Ki value of 0.2-0.3 mM. The natural racemic Gossypol has two enantiomers, namely the (-)-Gossypol and (+)-Gossypol enantiomers. The racemic form and each of the enantiomers of Gossypol are tested against UM-SCC-6 and UM-SCC-14A in 6-day MTT assays. (-)-Gossypol exhibits greater growth inhibition relative to (±)-Gossypol than (+)-Gossypol in both cell lines tested (P<0.001). An intermediate growth inhibitory effect is observed with (±)-Gossypol but this effect is only observed at the higher dose of Gossypol (10 μM, P<0.0001)[1]. |
| Cell Assay | Two representative UM-SCC cell lines, UM-SCC-6 and UM-SCC-14A, are continuously exposed to 0 (vehicle control), 5 or 10 μM (±)-Gossypol, (-)-Gossypol or (+)-Gossypol in a 6-day MTT cell survival assay[1]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 707.9±55.0 °C at 760 mmHg |
| Melting Point | 181-183ºC |
| Molecular Formula | C30H30O8 |
| Molecular Weight | 518.554 |
| Flash Point | 395.9±28.0 °C |
| Exact Mass | 518.194092 |
| PSA | 155.52000 |
| LogP | 6.16 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.742 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H351 |
| Precautionary Statements | P281 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn |
| Risk Phrases | R22;R40 |
| Safety Phrases | S22-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3.0 |
| RTECS | DU3100000 |
|
~77% 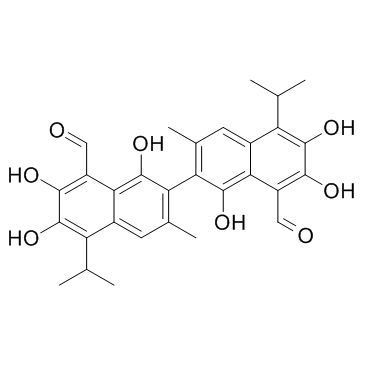
303-45-7 |
| Literature: Li, Ling; Liu, Yuxiu; Wang, Qingmin European Journal of Organic Chemistry, 2013 , # 35 p. 8014 - 8021 |
|
~73% 
303-45-7 |
| Literature: Meltzer; Bickford; Lambert Journal of Organic Chemistry, 1985 , vol. 50, # 17 p. 3121 - 3124 |
|
~% 
303-45-7 |
| Literature: European Journal of Organic Chemistry, , # 35 p. 8014 - 8021 |
|
~% 
303-45-7 |
| Literature: European Journal of Organic Chemistry, , # 35 p. 8014 - 8021 |
|
~% 
303-45-7 |
| Literature: European Journal of Organic Chemistry, , # 35 p. 8014 - 8021 |
|
~% 
303-45-7 |
| Literature: European Journal of Organic Chemistry, , # 35 p. 8014 - 8021 |
|
~% 
303-45-7 |
| Literature: Journal of the Chemical Society, Perkin Transactions 2: Physical Organic Chemistry (1972-1999), , # 9 p. 1359 - 1362 |
|
~% 
303-45-7 |
| Literature: Chemistry of Natural Compounds, , vol. 26, # 2 p. 129 - 138 Khimiya Prirodnykh Soedinenii, , # 2 p. 166 - 177 |
|
~% 
303-45-7 |
| Literature: Journal of Biological Chemistry, , vol. 76, p. 233 |
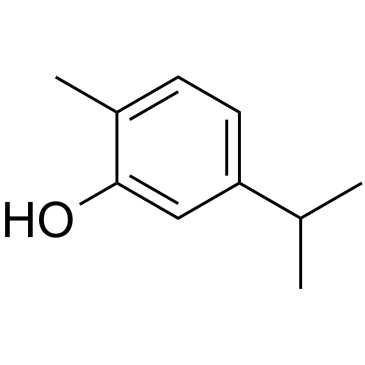
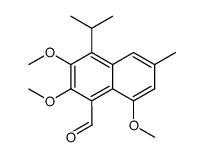
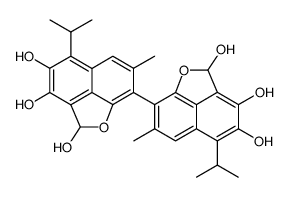
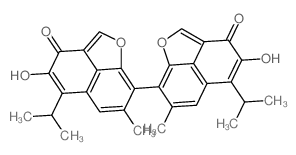
| Precursor 7 | |
|---|---|
| DownStream 8 | |





![1-Naphthalenecarboxaldehyde,7-[2,10-dihydroxy-5,13-dimethyl-3,11-bis(1-methylethyl)-1,9-dioxo-14-[1,6,7-trihydroxy-8-(iminomethyl)-3-methyl-5-(1-methylethyl)-2-naphthalenyl]-1H,9H-dinaphtho[1,8-bc:1', structure](https://image.chemsrc.com/caspic/159/21891-57-6.png)
![2,2',3,3',4,4'-hexamethoxy-7,7'-dimethyl-5,5'-di(propan-2-yl)-2h,2'h-8,8'-binaphtho[1,8-bc]furan structure](https://image.chemsrc.com/caspic/492/31591-07-8.png)