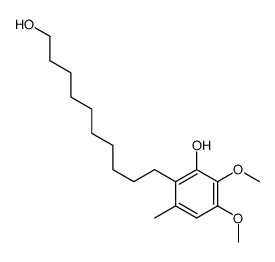
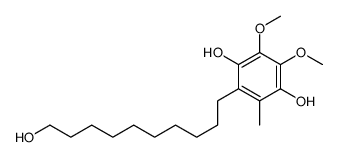
58186-27-9
| Name | idebenone |
|---|---|
| Synonyms |
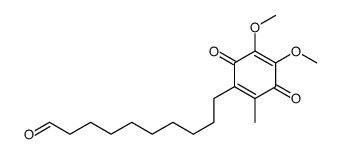
6-(10-Hydroxydecyl)ubiquinone
Raxone Idebenone [INN:JAN] Daruma Catena CV 2619 2-(10-Hydroxydecyl)-5,6-dimethoxy-3-methyl-1,4-benzoquinone Sovrima 2-(10-hydroxydecyl)-5,6-dimethoxy-3-methylbenzo-1,4-quinone Idebenone Idebenonum [Latin] 2-(10-hydroxydecyl)-5,6-dimethoxy-3-methyl-cyclohexa-2,5-diene-1,4-dione 2-(10-hydroxydecyl)-5,6-dimethoxy-3-methylcyclohexa-2,5-diene-1,4-dione MFCD00274552 UNII-HB6PN45W4J 2,3-Dimethoxy-5-methyl-6-(10'-hydroxydecyl)-1,4-benzoquinone 2-(10-Hydroxydecyl)-5,6-dimethoxy-3-methyl-2,5-cyclohexadiene-1,4-dione Lucenabol 2,5-Cyclohexadiene-1,4-dione, 2-(10-hydroxydecyl)-5,6-dimethoxy-3-methyl- Idebenone (JAN) Mnesis Avan Idebenona [Spanish] |
| Description | Idebenone is a synthetic variant of coenzyme Q10 (CoQ10), which initially developed for the treatment of Alzheimer's disease and other cognitive defects.Target: OthersIdebenone is a synthetic variant of coenzyme Q10 (CoQ10), which initially developed for the treatment of Alzheimer's disease and other cognitive defects. Idebenone was generally well tolerated with similar numbers of adverse events in each group. One child receiving high-dose idebenone developed neutropenia after 6 months, which resolved after discontinuation of treatment. 8OH2′dG concentrations were not increased, and did not significantly change with idebenone treatment [1]. The 2-year efficacy and safety of idebenone were studied in a prospective, randomized, double-blind multicentre study in 3 parallel groups of patients with dementia of the Alzheimer type (DAT) of mild to moderate degree. A total of 450 patients were randomized to either placebo for 12 months, followed by idebenone 90 mg tid for another 12 months (n = 153) or idebenone 90 mg tid for 24 months (n = 148) or 120 mg tid for 24 months (n = 149). idebenone showed statistically significant dose-dependent improvement in the primary efficacy variable ADAS-Total and in all the secondary efficacy variables [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 497.3±45.0 °C at 760 mmHg |
| Melting Point | 52-550C |
| Molecular Formula | C19H30O5 |
| Molecular Weight | 338.439 |
| Flash Point | 170.1±22.2 °C |
| Exact Mass | 338.209320 |
| PSA | 72.83000 |
| LogP | 3.49 |
| Vapour Pressure | 0.0±2.9 mmHg at 25°C |
| Index of Refraction | 1.502 |
| Storage condition | Room temp |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi: Irritant; |
|---|---|
| RIDADR | NONH for all modes of transport |
| RTECS | GU5290000 |
| Precursor 8 | |
|---|---|
| DownStream 2 | |