77671-31-9
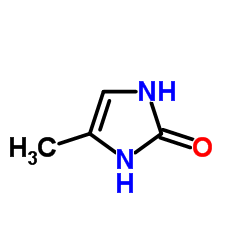
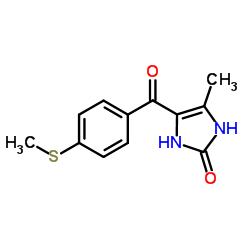
| Name | 4-methyl-5-(4-methylsulfanylbenzoyl)-1,3-dihydroimidazol-2-one |
|---|---|
| Synonyms |
Fenoximone
Enoximonum [Latin] 2H-Imidazol-2-one, 1,3-dihydro-4-methyl-5-(4-(methylthio)benzoyl)- 5-methyl-4-(4-(methylthio)benzoyl)-1H-imidazol-2(3H)-one [14C]-Enoximone 2H-Imidazol-2-one, 1,3-dihydro-4-methyl-5-[4-(methylthio)benzoyl]- 4-Methyl-5-[4-(methylsulfanyl)benzoyl]-1,3-dihydro-2H-imidazol-2-one Enoximona MFCD00867130 Perfane Enoximonum Enoximona [Spanish] 1,3-dihydro-4-methyl-5-[4-(methylthio)benzoyl]-2H-imidazol-2-one Enoximone Perfan |
| Description | Enoximone is an inotropic vasodilating agent and a selective and orally active phosphodiesterase III (PDE3) inhibitor with an IC50 of 5.9 μM. Enoximone induces vasodilatation and increases intracellular levels of cAMP by inhibiting cGMP-inhibited PDE. Enoximone also exhibits PDE4 inhibitory effect with an IC50 of 21.1 μM for myocardial PDE4A. Enoximone has the potential for congestive heart failure research and has bronchodilatory, antiasthma and anti-inflammatory effects[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
PDE3/PDE Ⅲ:5.9 μM (IC50) PDE4A:21.1 μM (IC50, myocardial PDE4A) |
| In Vitro | In vitro, 10 μM Enoximone-treated bronchoalveolar lavage (BAL) eosinophils induced by IL-33 treatment shows significantly lower CD11b expression when compared with diluent-treated BAL eosinophils[1]. |
| In Vivo | Topical Enoximone (25 μg; intratracheal route) abrogates house dust mite (HDM)-induced allergic airway inflammation[1]. The Enoximone-treated (25 μg; for 5 days) HDM-exposed mice shows significant reductions in inflammatory cell numbers including eosinophils, macrophages, neutrophils, ILC2s, and T cells, indicating that Enoximone treatment reduces airway inflammation[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Melting Point | 255-258°C |
| Molecular Formula | C12H12N2O2S |
| Molecular Weight | 248.301 |
| Exact Mass | 248.061951 |
| PSA | 91.02000 |
| LogP | 3.72 |
| Appearance | solid | light yellow |
| Index of Refraction | 1.645 |
| Storage condition | Store at +4°C |
| Water Solubility | DMSO: 28 mg/mL, soluble |
| Personal Protective Equipment | Eyeshields;Gloves;half-mask respirator (US);multi-purpose combination respirator cartridge (US) |
|---|---|
| RIDADR | 3249 |
| WGK Germany | 3 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933290090 |
|
~% 
77671-31-9 |
| Literature: US4405635 A1, ; |
|
~50% 
77671-31-9 |
| Literature: Schnettler; Dage; Grisar Journal of Medicinal Chemistry, 1982 , vol. 25, # 12 p. 1477 - 1481 |
|
~% 
77671-31-9 |
| Literature: Arkivoc, , vol. 2014, # 2 p. 294 - 307 |
|
~% 
77671-31-9 |
| Literature: Arkivoc, , vol. 2014, # 2 p. 294 - 307 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |