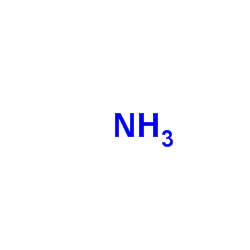
496-93-5
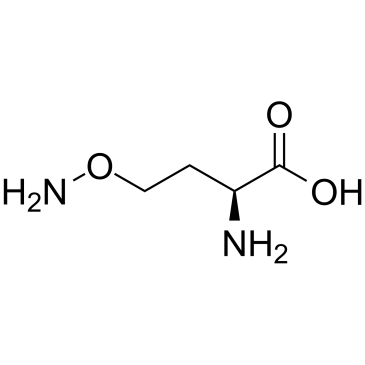
| Name | (2S)-2-amino-4-aminooxybutanoic acid |
|---|---|
| Synonyms |
L-2-amino-4-(aminooxy)butyric acid
O-Amino-homoserin Canaline O-amino-homoserine O-amino-DL-homoserine O-Amino-DL-homoserin 2-amino-4-aminooxybutanoic acid L-a-amino-g-(aminooxy)-n-butyric acid (S)-2-Amino-4-(aminooxy)butanoic acid L-2-amino-4-(aminooxy)butyrate L-Homoserine,O-amino O-Amino-L-homoserine L-Canaline |
| Description | L-Canaline is a nonprotein amino acid stored in many leguminous plants. L-Canaline is a cytotoxic metabolite catalyzed by L-canavanine and its arginase. L-Canaline is a potent and irreversible inhibitor of ornithine aminotransferase. L-Canaline inhibits the growth of the malaria parasite Plasmodium falciparum with an IC50 of 297 nM. L-Canaline has anticancer and antiproliferative effects[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 297 nM (Plasmodium falciparum)[3]; Ornithine aminotransferase[1] |
| In Vitro | L-Canaline treatment inhibits the proliferation of PBMCs after stimulation by phorbol 12-myristate-13-acetate (PMA) or via the mixed lymphocyte reaction. The greatest effect is seen with PMA-stimulated cells, where L-canaline has an IC50 of 0.26 mM. L-Canaline is slightly less toxic to PBMCs stimulated via the mixed lymphocyte reaction (IC50 of 0.54 mM)[1].L-canaline inhibits L-lysine flux competitively (Ki of 4.6 mM) in astrocytes and astrocytoma cells[2]. |
| In Vivo | L-Canaline decreases the aspartic acid content of tissues of the medulla oblongata of male Wistar rats, but it does not affect the evoked release of this nonprotein amino acid into these tissues[2]. Intraseptal injection of 100 μg of L-canaline into male Sprague-Dawley rats causes a 90% decrease in the omithine aminotransferase activity of the septum tissues evaluated from animals killed 1 h later[2]. |
| References |
| Density | 1.298g/cm3 |
|---|---|
| Boiling Point | 378.1ºC at 760mmHg |
| Molecular Formula | C4H10N2O3 |
| Molecular Weight | 134.13400 |
| Flash Point | 182.5ºC |
| Exact Mass | 134.06900 |
| PSA | 98.57000 |
| LogP | 0.07930 |
| Vapour Pressure | 9.22E-07mmHg at 25°C |
| Index of Refraction | 1.51 |
| Hazard Codes | Xi |
|---|---|
| Safety Phrases | S22-S24/25 |
| HS Code | 2922509090 |
|
~% 
496-93-5 |
| Literature: Nyberg; Christensen Journal of the American Chemical Society, 1957 , vol. 79, p. 1222,1225 |
|
~% 
496-93-5 |
| Literature: Gilon,C. et al. Tetrahedron, 1967 , vol. 23, p. 4441 - 4447 |
|
~% 
496-93-5 |
| Literature: Knobler; Frankel Journal of the Chemical Society, 1958 , p. 1629 |
|
~% 
496-93-5 |
| Literature: SCHEBO.BIOTECH AG Patent: WO2005/54174 A2, 2005 ; Location in patent: Page/Page column 17-18 ; |
| Precursor 3 | |
|---|---|
| DownStream 2 | |
| HS Code | 2922509090 |
|---|---|
| Summary | 2922509090. other amino-alcohol-phenols, amino-acid-phenols and other amino-compounds with oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
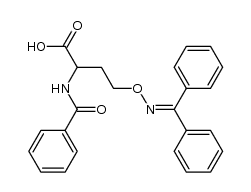
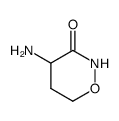
![2-[N-(benzenesulfonyl)-4-propan-2-ylanilino]-N-(4-methoxyphenyl)acetamide structure](https://www.chemsrc.com/caspic/174/6169-65-9.png)