94-62-2
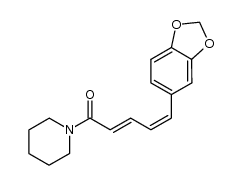
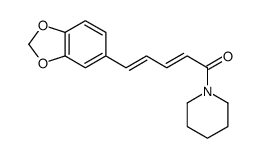
| Name | piperine |
|---|---|
| Synonyms |
2,4-Pentadien-1-one, 5-(1,3-benzodioxol-5-yl)-1-(1-piperidinyl)-, (2E,4E)-
(2E,4E)-5-(1,3-Benzodioxol-5-yl)-1-(1-piperidinyl)-2,4-pentadien-1-one 5-(1,3-benzodioxol-5-yl)-1-piperidinopenta-2,4-dien-1-one (2E,4E)-5-(1,3-Benzodioxol-5-yl)-1-(piperidin-1-yl)penta-2,4-dien-1-one UNII:U71XL721QK (E,E)-1-[5-(1,3-Benzodioxol-5-yl)-1-oxo-2,4-pentadienyl]piperidine 1-(5-(3,4-Methylenedioxyphenyl)-1-oxo-2,4-pentadienyl)piperidine trans,trans-1-piperoylpiperidine FEMA 2909 1-PIPEROYL-(E,E)-PIPERIDINE Piperine 1-Piperoylpiperidine, (E,E)- Piperidine, 1-[5- (1,3-benzodioxol-5-yl)-1-oxo-2,4-pentadienyl]- piperidine, 1-[(2E,4E)-5-(1,3-benzodioxol-5-yl)-1-oxo-2,4-pentadienyl]- Piperidine, 1-piperoyl-, (E,E)- (E,E)-1-piperoylpiperidine Piperidine, 1-(5-(3,4-methylenedioxyphenyl)-1-oxo-2,4-pentadienyl)- MFCD00005839 PIPERLINE Piperidine, 1-(5-(1,3-benzodioxol-5-yl)-1-oxo-2,4-pentadienyl)- Piperidine, 1-[5-(1,3-benzodioxol-5-yl)-1-oxo-2,4-pentadienyl]-, (E,E)- Piperin 1-Piperoylpiperidine EINECS 202-348-0 Bioperine |
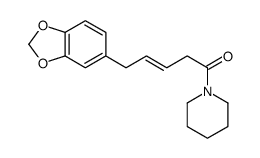
| Description | Piperine, a natural alkaloid isolated from Piper nigrum L, inhibits P-glycoprotein and CYP3A4 activities with an IC50 value of 61.94±0.054 μg/mL in HeLa cell. |
|---|---|
| Related Catalog | |
| Target |
IC50: 61.94±0.054 μg/mL (P-glycoprotein, HeLa cell)[1] |
| In Vitro | Piperine has shown to possess in vitro cytotoxic activity and in silico studies. The IC50 value is found to be 61.94±0.054 μg/mL and in silico studies, it has more number of hydrogen bonds with minimum binding and docking energy and may be considered as inhibitor of EGFR tyrosine kinase[1]. Piperine has been found to have immunomodulatory, anti-oxidant, anti-asthmatic, anti-carcinogenic, anti-inflammatory, anti-ulcer, and anti-amoebic properties[2]. Piperine could enhance the bioavailabilities of other drugs including rosuvastatin, peurarin and docetaxel (DOX) via inhibition of CYP3A and P-glycoprotein activity[3]. |
| In Vivo | At the dose of 3.5 mg/kg, the bioavailability of piperine is calculated to be 25.36%. Its AUC0→t is unproportionally increased with doses, indicating a potential non-linear pharmacokinetics profile of piperine. It is found that the AUC0→t and C0 of docetaxel and t1/2of piperine are significantly increased after their combination use, suggesting potential enhanced bioavailability of not only docetaxel but also piperine, which may lead to the overall enhanced pharmacological effects[3]. The phosphorylation of I-κB, p65, p38, ERK, and JNK is inhibited by piperine in a dose-dependent manner, indicating that piperine may be a potential anti-inflammatory drug both in endometritis and in other S. aureus-induced diseases[4]. |
| Cell Assay | Standard solution is prepared by dissolving 10 mg of piperine in 100 mL of methanol. The MTT assay is carried out to measure cell viability. Ten thousand cells in 100 μL of DMEM media are seeded in the wells of a 96-well plate. After 24 h, existing media is removed and 100 μL of various concentrations of piperine (20–100 μg/mL) are added and incubated for 48 h at 37 °C in a CO2 incubator. Control cells are supplemented with 0.05 % DMSO vehicle. At the 48th hour of incubation, MTT (10 μL of 5 mg/mL) is added to the plate. The contents of the plate are pipetted out carefully, the formazan crystals formed are dissolved in 100 μL of DMSO, and the absorbance is measured at 550 nm in a microplate reader[1]. |
| Animal Admin | Rats: The stock solutions of piperine (PIP) and docetaxel (DOX)are prepared by dissolving appropriate amount of each authentic compound in DMSO separately at 1 mg/mL. The standard solutions containing both PIP and DOX are prepared by serial dilution of the stock solutions with 0.2% formic acid and acetonitrile (50:50, v/v) to yield concentrations of 25, 50, 100, 200, 400, 800, 1600, 3200, 6400, 12800 ng/mL. 25 Sprague-Dawley rats are divided into five groups receiving DOX(Group DOX 7 iv, 7 mg/kg, i.v.), PIP (Group PIP 35 po, 35 mg/kg,p.o.) and their combined administration (Group DOX+PIP) as well as PIP (Group PIP 3.5 po, 3.5 mg/kg, p.o.) and PIP (Group PIP 3.5 iv, 3.5 mg/kg, i.v.)[3]. Mice: Piperine is dissolved in 5 mL of tris buffered saline (TBS) at concentrations corresponding to 25, 50, and 100 mg/kg, based on the weight of the mice. After 24 h of S. aureus infection in the uterus, the piperine solution is injected intraperitoneally three times every 6 h. A total of 60 female BALB/c mice are used in this study. All mice are maintained on a 12 h light/dark cycle and cafeteria feeding[4]. |
| References |
[2]. Meghwal M,et al. Piper nigrum and piperine: an update. Phytother Res. 2013 Aug;27(8):1121-30. |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 498.5±40.0 °C at 760 mmHg |
| Melting Point | 131-135 °C(lit.) |
| Molecular Formula | C17H19NO3 |
| Molecular Weight | 285.338 |
| Flash Point | 255.3±27.3 °C |
| Exact Mass | 285.136505 |
| PSA | 38.77000 |
| LogP | 2.66 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.615 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| Water Solubility | 40 mg/L (18 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn,Xi |
| Risk Phrases | R21/22 |
| Safety Phrases | S22-S24/25-S36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | TN2321500 |
| HS Code | 2939999090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |


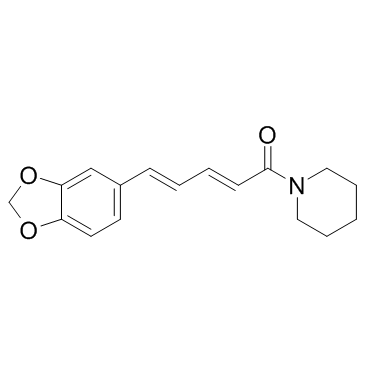

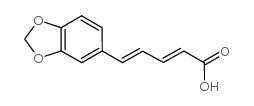
![1-[(E)-3,4-(methylenedioxy)cinnamylsulfonyl]acetyl-piperidine structure](https://image.chemsrc.com/caspic/246/736947-76-5.png)
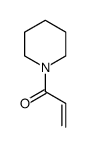
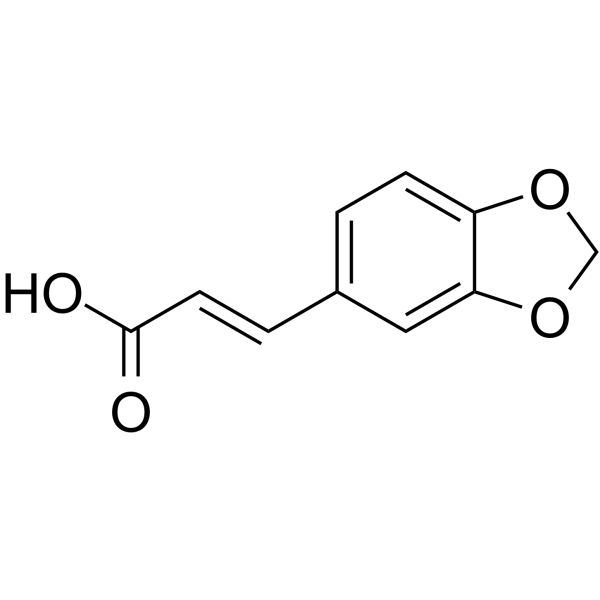
![(3R,6S)-diethyl 3-(benzo[d][1,3]dioxol-5-yl)-6-(piperidine-1-carbonyl)-2H-selenopyran-2,2(3H,6H)-dicarboxylate structure](https://image.chemsrc.com/caspic/397/139059-85-1.png)