112093-28-4
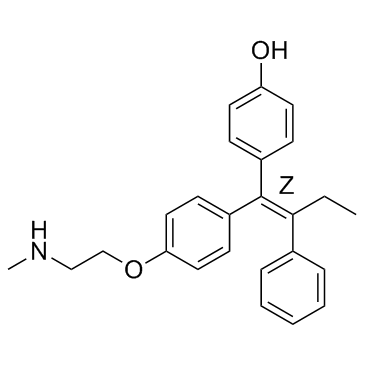
| Name | 4-[(Z)-1-[4-[2-(methylamino)ethoxy]phenyl]-2-phenylbut-1-enyl]phenol |
|---|---|
| Synonyms |
Z-Endoxifen
4OHNDtam 4-Hydroxy-N-desmethyltamoxifen Endoxifen UNII-46AF8680RC Endoxifen (Z-isomer) |
| Description | Endoxifen Z-isomer is the most important Tamoxifen metabolite responsible for eliciting the anti-estrogenic effects of this drug in breast cancer cells expressing estrogen receptor-alpha (ERα). Endoxifen inhibits hERG tail currents at 50 mV in a concentration-dependent manner with IC50 values of 1.6 μM.IC50 value: 1.6 μM [1]Target: hERG Potassium Channel, Estrogen Receptor/ERREndoxifen Z-isomer is considered a prodrug, since it has a much higher potency for the estrogen receptor than its parent drug. Endoxifen inhibits the hERG channel protein trafficking to the plasma membrane in a concentration-dependent manner with Endoxifen being more potent than Tamoxifen. [1] Endoxifen is also shown to be a more potent inhibitor of estrogen target genes when ERβ is expressed. Additionally, low concentrations of Endoxifen Z-isomer observed in Tamoxifen treated patients with deficient CYP2D6 activity (20 to 40 nM) markedly inhibit estrogen-induced cell proliferation rates in the presence of ERβ, whereas much higher Endoxifen Z-isomer concentrations are needed when ERβ is absent.[2] |
|---|---|
| Related Catalog | |
| References |
| Density | 1.099g/cm3 |
|---|---|
| Boiling Point | 519.327ºC at 760 mmHg |
| Melting Point | 127-129°C |
| Molecular Formula | C25H27NO2 |
| Molecular Weight | 373.48700 |
| Flash Point | 267.88ºC |
| Exact Mass | 373.20400 |
| PSA | 41.49000 |
| LogP | 5.75040 |
| Vapour Pressure | 0mmHg at 25°C |
| Index of Refraction | 1.599 |
| Storage condition | Amber Vial, -86°C Freezer, Under Inert Atmosphere |
| Symbol |

GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H400 |
| Precautionary Statements | P273-P391 |
| RIDADR | UN 3077 9 / PGIII |
| RTECS | SL8285000 |