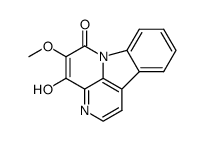
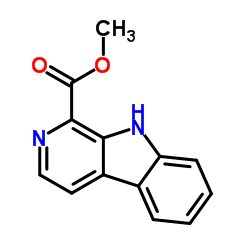
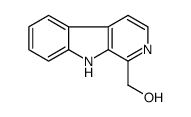
18110-87-7
| Name | 4,5-Dimethoxycanthin-6-one |
|---|---|
| Synonyms |
Nigakinon-methylether
Methyl nigakinone 4,5-Dimethoxy-canthin-6-on 4,5-Dimethoxy-6H-indolo[3,2,1-de][1,5]naphthyridin-6-one 6H-Indolo(3,2,1-de)(1,5)naphthyridin-6-one, 4,5-dimethoxy- 6H-Indolo[3,2,1-de][1,5]naphthyridin-6-one, 4,5-dimethoxy- 4,5-dimethoxy-6H-indolo[3,2,1-de]-1,5-naphthyridin-6-one 4,5-Dimethoxy-6-oxo-6H-indolo<3.2.1-de><1.5>naphthyridin |
| Description | 4,5-Dimethoxycanthin-6-one is a potent and uncompetitive inhibitor of CYP1A2-mediated phenacetin O-deethylation with an IC50 value of 1.7μM and a Ki value of 2.6 μM. 4,5-Dimethoxycanthin-6-one, as an alkaloid, is isolated from the wood of Picrasma quassioides BENNET (Simaroubaceae)[1][2]. |
|---|---|
| Related Catalog | |
| Target |
CYP1A2:1.7 μM (IC50) CYP1A2:2.6 μM (Ki) |
| In Vitro | 4,5-Dimethoxycanthin-6-one is a potent and uncompetitive inhibitor of CYP1A2-mediated phenacetin O-deethylation with an IC50 value of 1.7μM and a Ki value of 2.6 μM[2]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 455.1±45.0 °C at 760 mmHg |
| Melting Point | 145-146 °C |
| Molecular Formula | C16H12N2O3 |
| Molecular Weight | 280.278 |
| Flash Point | 229.0±28.7 °C |
| Exact Mass | 280.084778 |
| PSA | 52.83000 |
| LogP | 2.16 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.688 |
| Hazard Codes | Xi |
|---|
|
~% 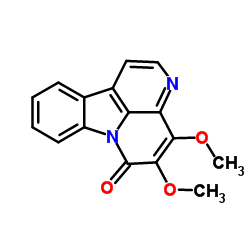
18110-87-7 |
| Literature: Ohmoto, Taichi; Koike, Kazuo Chemical & Pharmaceutical Bulletin, 1984 , vol. 32, # 9 p. 3579 - 3583 |
|
~% 
18110-87-7 |
| Literature: Ohmoto; Koike Chemical and Pharmaceutical Bulletin, 1985 , vol. 33, # 11 p. 4901 - 4905 |
| Precursor 2 | |
|---|---|
| DownStream 2 | |