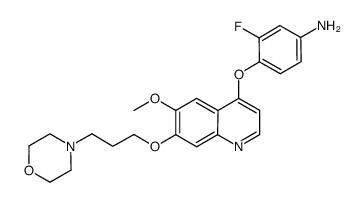
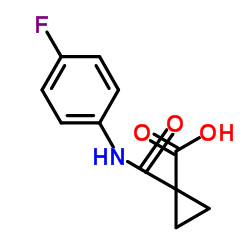
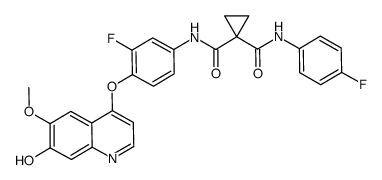
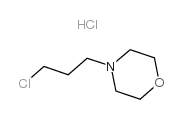
849217-64-7
| Name | N-(3-Fluoro-4-((6-methoxy-7-(3-morpholinopropoxy)quinolin-4-yl)-oxy)phenyl)-N-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide |
|---|---|
| Synonyms |
4-(4-(o-Methoxyphenyl)piperazinyl)-1-(3,4,5-trimethoxyphenyl)-1-butanol dihydrochloride
1-Butanol,4-(4-(o-methoxyphenyl)piperazinyl)-1-(3,4,5-trimethoxyphenyl)-,dihydrochloride 4-[4-(2-methoxyphenyl)piperazin-1-yl]-1-(3,4,5-trimethoxyphenyl)butan-1-ol dihydrochloride 1,1-Cyclopropanedicarboxamide, N-[3-fluoro-4-[[6-methoxy-7-[3-(4-morpholinyl)propoxy]-4-quinolinyl]oxy]phenyl]-N'-(4-fluorophenyl)- EXEL-2880 4-(4-Hydroxy-4-(3,4,5-trimethoxyphenyl)butyl)-1-(o-methoxyphenyl)piperazine dihydrochloride N-[3-fluoro-4-([6-(methyloxy)-7-[(3-morpholin-4-ylpropyl)oxy]quinolin-4-yl]oxy)phenyl]-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide Foretinib N1-{3-fluoro-4-[(6-(methyloxy)-7-{[3-(4-morpholinyl)propyl]oxy}-4-quinolinyl)oxy]phenyl}-N1'-(4-fluorophenyl)-1,1-cyclopropanedicarboxamide N-[3-Fluoro-4-({6-methoxy-7-[3-(4-morpholinyl)propoxy]-4-quinolinyl}oxy)phenyl]-N'-(4-fluorophenyl)-1,1-cyclopropanedicarboxamide |
| Description | Foretinib is a multi-target tyrosine kinase inhibitor with IC50s of 0.4 nM and 0.9 nM for Met and KDR. |
|---|---|
| Related Catalog | |
| Target |
KDR:0.9 nM (IC50) c-Met:0.4 nM (IC50) |
| In Vitro | Foretinib inhibits HGF receptor family tyrosine kinases with IC50 values of 0.4 nM for Met and 3 nM for Ron. Foretinib also inhibits KDR, Flt-1, and Flt-4 with IC50 values of 0.9 nM, 6.8 nM and 2.8 nM, respectively. Foretinib inhibits colony growth of B16F10, A549 and HT29 cells with IC50 of 40 nM, 29 nM and 165 nM, respectively[1]. A recent study indicates Foretinib affects cell growth differently in gastric cancer cell lines MKN-45 and KATO-III. Foretinib inhibits phosphorylation of MET and downstream signaling molecules in MKN-45 cells, while targets GFGR2 in KATO-III cells[2]. |
| In Vivo | Foretinib (100 mg/kg, p.o.) results in substantial inhibition of phosphorylation of B16F10 tumor Met and ligand (e.g., HGFor VEGF)-induced receptor phosphorylation of Met in liver and Flk-1/KDR in lung, which both persist through 24 hours. Foretinib (30-100 mg/kg, once daily, p.o.) results in reduction in tumor burden. The lung surface tumor burden is reduced by 50% and 58% following treatment with 30 and 100 mg/kg Foretinib, respectively. Foretinib treatment of mice bearing B16F10 solid tumors also results in dose-dependent tumor growth inhibition of 64% and 87% at 30 and 100 mg/kg, respectively. For both studies, administration of Foretinib is well tolerated with no significant body weight loss[1]. Foretinib is developed to target abnormal signaling of HGF through Met and simultaneously target several receptors tyrosine kinase involved in tumor angiogenesis. Foretinib causes tumor hemorrhage and necrosis in human xenografts within 2 to 4 hours, and maximal tumornecrosis is observed at 96 hours (after five daily doses), resulting in complete regression[3]. |
| Kinase Assay | Kinase inhibition is investigated using one of three assay formats: [33P]phosphoryl transfer, luciferase-coupled chemiluminescence, or AlphaScreen tyrosine kinase technology. IC50s are calculated by nonlinear regression analysis using XLFit.33P -Phosphoryl Transfer Kinase Assay Reactions are performed in 384-well white, clear bottom, high-binding microtiter plates. Plates are coated with 2 μg/well of protein or peptide substrate in a 50 μL volume of coating buffer contained 40 μg/mL substrate (poly(Glu, Tyr) 4:1, 22.5 mM Na2CO3, 27.5 mM NaHCO3, 50 mM NaCl and 3 mM NaN3. Coated plates are washed once with 50 μL of assay buffer following overnight incubation at room temperature (RT). Test compounds and enzymes are combined with 33P-γ-ATP (3.3 μCi/nmol) in a total volume of 20 μL. The reaction mixture is incubated at RT for 2 hours and terminated by aspiration. The microtiter plates are subsequently washed 6 times with 0.05% Tween-PBS buffer (PBST). Scintillation fluid (50 μL/well) is added and incorporated 33P is measured by liquid scintillation spectrometry using a MicroBeta scintillation counter.Luciferase-Coupled Chemiluminescence Assay Reactions are conducted in 384-well white, medium binding microtiter plates. In a first step enzyme and compound are combined and incubated for 60 minutes; reactions are initiated by addition of ATP and peptide substrate (poly(Glu, Tyr) 4:1) in a final voume of 20 μL, and incubated at RT for 2-4 hours. Following the kinase reaction, a 20 μL aliquot of Kinase Glo is added and luminescence signal is measured using a Victor plate reader. Total ATP consumption is limited to 50%. AlphaScreenTM Tyrosine Kinase Assay Donor beads coated with streptavidin and acceptor beads coated with PY100 anti-phosphotyrosine antibody are used. Biotinylated poly(Glu,Tyr) 4:1 is used as the substrate. Substrate phosphorylation is measured by addition of donor/acceptor beads by luminescence following donor-acceptor bead complex formation. Kinase and test compounds are combined and preincubated for 60 minutes, followed by addition of ATP, and biotinylated poly(Glu, Tyr) in a total volume of 20 μL in 384-well white, medium binding microtiter plates. Reaction mixtures are incubated for 1 hour at room temperature. Reactions are quenched by addition of 10 μL of 15-30 μg/mL AlphaScreen bead suspension containing 75 mM Hepes, pH 7.4, 300 mM NaCl, 120 mM EDTA, 0.3% BSA and 0.03% Tween-20. After 2-16 hours incubation at room temperature plates are read using an AlphaQuest reader. |
| Cell Assay | B16F10, A549, and HT29 cells (1.2×103 per well) are mixed with soft agar and seeded in a 96-well plate containing 10% FBS and EXEL-2880 over a base agar layer. For normoxic conditions, the plates are incubated (37°C) for 12 to 14 days in 21% oxygen, 5% CO2, and 74% nitrogen, whereas incubation (37°C) under hypoxic conditions is done in a hypoxia chamber in 1% oxygen, 5% CO2, and 94% nitrogen. The number of colonies is evaluated under each condition following addition of 50% Alamar Blue and fluorescence detection. |
| Animal Admin | In vivo target modulation studies are done in naive mice or mice bearing B16F10 tumors. Foretinib or vehicle (0.9% normal saline) is administered at 10 mL/kg via oral gavage. For examination of Met phosphorylation in liver, HGF (10 μg/mouse) is administered i.v. 10 min before harvest. For examination of Flk-1/KDR phosphorylation in lung, VEGF (10 μg/mouse) is administered i.v. 30 min before harvest 0.5 h later. Receptor phosphorylation analysis is determined by immunoblot analysis. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 828.5±65.0 °C at 760 mmHg |
| Molecular Formula | C34H34F2N4O6 |
| Molecular Weight | 632.654 |
| Flash Point | 454.8±34.3 °C |
| Exact Mass | 632.244629 |
| PSA | 116.45000 |
| LogP | 5.12 |
| Vapour Pressure | 0.0±3.0 mmHg at 25°C |
| Index of Refraction | 1.649 |
| Storage condition | -20°C |
|
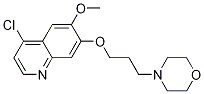
~86% 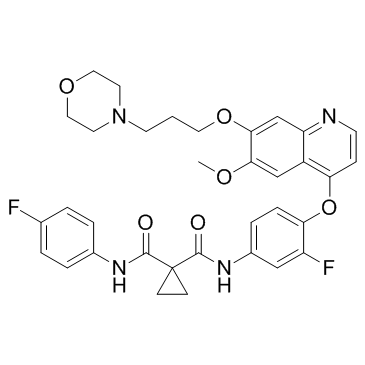
849217-64-7 |
| Literature: WO2011/9095 A1, ; Page/Page column 18-20 ; WO 2011/009095 A1 |
|
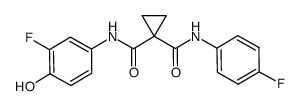
~70% 
849217-64-7 |
| Literature: WO2005/30140 A2, ; Page/Page column 209 ; WO 2005/030140 A2 |
|
~81% 
849217-64-7 |
| Literature: US2010/81805 A1, ; Page/Page column 13-14 ; |
| Precursor 4 | |
|---|---|
| DownStream 0 | |