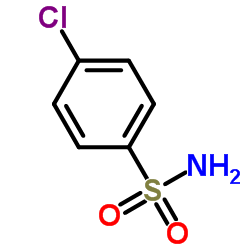
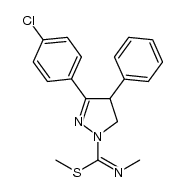
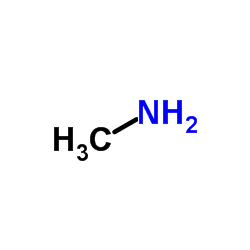
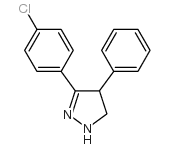
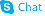362519-49-1
| Name | 5-(4-chlorophenyl)-N-(4-chlorophenyl)sulfonyl-N'-methyl-4-phenyl-3,4-dihydropyrazole-2-carboximidamide |
|---|---|
| Synonyms |
Ibipinabant
1H-Pyrazole-1-carboximidamide, 3-(4-chlorophenyl)-N'-[(4-chlorophenyl)sulfonyl]-4,5-dihydro-N-methyl-4-phenyl- 3-(4-chlorophenyl)-N-methyl-N'-((4-chlorophenyl)sulfonyl)-4-phenyl-4,5-dihydro-1H-pyrazole-1-carboxamidine 3-(4-Chlorophenyl)-N-[(4-chlorophenyl)sulfonyl]-N'-methyl-4-phenyl-4,5-dihydro-1H-pyrazole-1-carboximidamide 1H-pyrazole-1-carboximidamide, 3-(4-chlorophenyl)-N-[(4-chlorophenyl)sulfonyl]-4,5-dihydro-N'-methyl-4-phenyl- (±)-SLV319 |
| Description | (±)-SLV319 is the racemate of SLV319. SLV319 is a potent and selective cannabinoid-1 (CB-1) receptor antagonist with an IC50 of 22 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 22 nM (CB-1)[1]; Ki: 7.8 nM (CB-1)[2] |
| In Vitro | Cannabinoid receptor 1 (CB1R) antagonists appear to be promising drugs for the treatment of obesity, however, serious side effects have hampered their clinical application. Ibipinabant is a new, potent [Ki (CB1)=7.8 nM] and selective [Ki (CB2)=7.943 nM] CB1 antagonist [pA2 for arachidonic acid release in CHO cells=9.9] with in vitro pharmacological characteristics similar to rimonabant including inverse agonism and brain penetrance[3]. |
| In Vivo | Ibipinabant (3 mg/kg) reduces unfasted glucose to a significantly greater degree than rimonabant at the same dose on days 17, 28 and 38. Chronic treatment with ibipinabant significantly attenuates the progression of diabetes in ZDF rats, blunting the increase in blood glucose and HbA1c over time. Ibipinabant also reduces the hyperinsulinemia apparent at 6-8 weeks of age and attenuates the dramatic reduction in insulin levels observed 1-2 weeks later[3]. |
| Animal Admin | Rats: SLV319, rimonabant and rosiglitazone are suspended in a 10% dimethylacetamide, 10% cremophor, 10% ethanol and 70% water vehicle. Drugs are administered by oral gavage in a volume of 2 mL/kg body weight at 09:00 hours every day. Treatment groups are as follows: (i) Vehicle: ad libitum access to food (vehicle), (ii) Vehicle: restricted access to food (20% less than average food intake of ad libitum vehicle-treated group for the first 3 days of the study, then 10% less than the average food intake of the ad libitum vehicle-treated group for the remainder of the study) (restricted), (iii) Rosiglitazone (4 mg/kg), (iv) Rimonabant (3 mg/kg) (RIM 3 mg/kg), (v) Rimonabant (10 mg/kg) (RIM 10 mg/kg), (vi) SLV319 (3 mg/kg) (IBI 3 mg/kg) and (vii) Ibipinabant (10 mg/kg) (IBI 10 mg/kg). Rosiglitazone is used as a positive control for its ability to delay β-cell decline, and rimonabant is used as a positive control for CB1 antagonism[3]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 623.2±65.0 °C at 760 mmHg |
| Molecular Formula | C23H20Cl2N4O2S |
| Molecular Weight | 487.401 |
| Flash Point | 330.7±34.3 °C |
| Exact Mass | 486.068390 |
| PSA | 82.51000 |
| LogP | 4.67 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.663 |
|
~89% 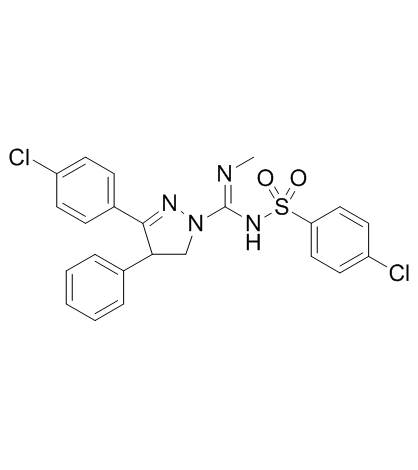
362519-49-1 |
| Literature: Lange, Jos H.M.; Sanders, Hans J.; Van Rheenen, Jeroen Tetrahedron Letters, 2011 , vol. 52, # 12 p. 1303 - 1305 |
|
~% 
362519-49-1 |
| Literature: Lange, Jos H. M.; Coolen, Hein K. A. C.; Van Stuivenberg, Herman H.; Dijksman, Jessica A. R.; Herremans, Arnoud H. J.; Ronken, Eric; Keizer, Hiskias G.; Tipker, Koos; McCreary, Andrew C.; Veerman, Willem; Wals, Henri C.; Stork, Bob; Verveer, Peter C.; Den Hartog, Arnold P.; De Jong, Natasja M. J.; Adolfs, Tiny J. P.; Hoogendoorn, Jan; Kruse, Chris G. Journal of Medicinal Chemistry, 2004 , vol. 47, # 3 p. 627 - 643 |
|
~% 
362519-49-1 |
| Literature: Lange, Jos H. M.; Coolen, Hein K. A. C.; Van Stuivenberg, Herman H.; Dijksman, Jessica A. R.; Herremans, Arnoud H. J.; Ronken, Eric; Keizer, Hiskias G.; Tipker, Koos; McCreary, Andrew C.; Veerman, Willem; Wals, Henri C.; Stork, Bob; Verveer, Peter C.; Den Hartog, Arnold P.; De Jong, Natasja M. J.; Adolfs, Tiny J. P.; Hoogendoorn, Jan; Kruse, Chris G. Journal of Medicinal Chemistry, 2004 , vol. 47, # 3 p. 627 - 643 |
|
~% 
362519-49-1 |
| Literature: Lange, Jos H. M.; Coolen, Hein K. A. C.; Van Stuivenberg, Herman H.; Dijksman, Jessica A. R.; Herremans, Arnoud H. J.; Ronken, Eric; Keizer, Hiskias G.; Tipker, Koos; McCreary, Andrew C.; Veerman, Willem; Wals, Henri C.; Stork, Bob; Verveer, Peter C.; Den Hartog, Arnold P.; De Jong, Natasja M. J.; Adolfs, Tiny J. P.; Hoogendoorn, Jan; Kruse, Chris G. Journal of Medicinal Chemistry, 2004 , vol. 47, # 3 p. 627 - 643 |
|
~% 
362519-49-1 |
| Literature: Lange, Jos H.M.; Sanders, Hans J.; Van Rheenen, Jeroen Tetrahedron Letters, 2011 , vol. 52, # 12 p. 1303 - 1305 |
|
~% 
362519-49-1 |
| Literature: Lange, Jos H.M.; Sanders, Hans J.; Van Rheenen, Jeroen Tetrahedron Letters, 2011 , vol. 52, # 12 p. 1303 - 1305 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |