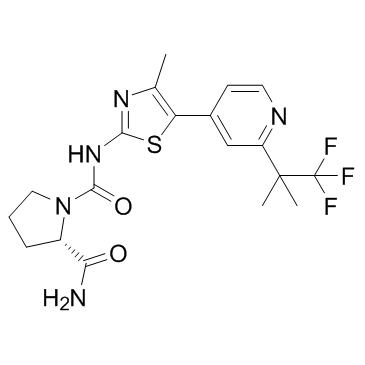
1217486-61-7
| Name | (2S)-1-N-[4-methyl-5-[2-(1,1,1-trifluoro-2-methylpropan-2-yl)pyridin-4-yl]-1,3-thiazol-2-yl]pyrrolidine-1,2-dicarboxamide |
|---|---|
| Synonyms |
UNII:08W5N2C97Q
1,2-Pyrrolidinedicarboxamide, N-[4-methyl-5-[2-(2,2,2-trifluoro-1,1-dimethylethyl)-4-pyridinyl]-2-thiazolyl]-, (2S)- QCR-1 BYL719 (S)-pyrrolidine-1,2-dicarboxylic acid 2-amide 1-{4-methyl-5-[2-(2,2,2-trifluoro-1,1-dimethyl-ethyl)-pyridin-4-yl]-thiazol-2-yl}amide (2S)-N-{4-Methyl-5-[2-(1,1,1-trifluoro-2-methyl-2-propanyl)-4-pyridinyl]-1,3-thiazol-2-yl}-1,2-pyrrolidinedicarboxamide Alpelisib (2S)-N1 -{4-methyl-5-[1-(1,1,1-trifluoro-2-methylpropan-2-yl)pyridin- 4-yl]-1,3-thiazol-2-yl}pyrrolidine-1,2-dicarboxamide BYL-719 |
| Description | Alpelisib (BYL-719) is a potent and selective PI3Kα inhibitor with an IC50 of 5 nM. |
|---|---|
| Related Catalog | |
| Target |
p110α:5 nM (IC50) p110γ:250 nM (IC50) p110δ:290 nM (IC50) p110β:1200 nM (IC50) |
| In Vitro | Alpelisib (NVP-BYL719) potently inhibits the 2 most common PIK3CA somatic mutations (H1047R, E545K; IC50~4 nM). Alpelisib (NVP-BYL719) potently inhibits Akt phosphorylation in cells transformed with PI3Kα (IC50=74±15 nM) and shows significant reduced inhibitory activity in PI3Kβ or PI3Kδ isoforms transformed cells (≥15-fold compared with PI3Kα)[2]. Alpelisib (NVP-BYL719) decreases cell proliferation by blocking cell cycle in G0/G1 phase with no outstanding effects on apoptosis cell death in HOS and MOS-J tumor cells. BYL-719 inhibits cell migration and can thus be considered as a cytostatic drug for osteosarcoma. In murine preclinical models of osteosarcoma, Alpelisib (NVP-BYL719) significantly decreases tumor progression and tumor ectopic bone formation as shown by a decrease of Ki67+ cells and tumor vascularization. Alpelisib (NVP-BYL719) rapidly inhibits the levels of P-AKT and P-mTOR in all cell lines assessed, confirming the functional activity of Alpelisib (NVP-BYL719) on osteosarcoma cells. After 72 hr of treatment, Alpelisib (NVP-BYL719) significantly inhibits the cell growth of all osteosarcoma cell lines tested in a dose-dependent manner with an IC50 ranging from 6 to 15 µM and with the IC90 from 24 to 42 µM at 72 hr[3]. |
| In Vivo | Alpelisib (BYL-719) displays excellent oral bioavailability in rats, mice and dogs and does not show any significant inhibition of the CYP450 enzymes[1]. Alpelisib (BYL-719) inhibits tumor growth in pre-clinical murine models of osteosarcoma. C57Bl/6J with MOS-J tumors (n=6 per group) are randomized as controls (vehicle) or Alpelisib (BYL-719) (12.5 mg/kg or 50 mg/kg per day)[3]. |
| Cell Assay | Two thousand tumor cells are seeded into 96-well plates and, the day after, the cells are treated with Alpelisib (BYL-719) (1-50 µM) for 72 hr. Cell growth/viability is determined using a colorimetric assay using sodium 3′[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro-)benzene sulfonic acid hydrate (XTT Reagent Assay Kit). Absorbance is read at 490 nm. Cell viability is also determined by trypan blue exclusion assay; viable and nonviable cells are counted manually after 24 and 48 hr of treatment[3]. |
| Animal Admin | Rats[2] Tumor xenografts are grown subcutaneously or orthotopically in nude mice or nude Rowett rats (Hsd: RH-Fox1rnu) by injection of 3×106 to 1×10 7 cells or implantation of tumor fragments of approximately 50 mg. Tumor-bearing animals mice are treated with either vehicle control, Alpelisib (NVP-BYL719), or NVP-BKM120 (p.o., every day) at the doses indicated. For efficacy studies, tumor-bearing animals are enrolled when subcutaneously implanted tumors reached about 200 mm3 and treated with Alpelisib (NVP-BYL719) at 50 mg/kg daily. The response is reported as percentage change in tumor volume at last day of treatment relative to day 0 (start of treatment). Mice[3] A 5-week-old male C57Bl/6J mice are anesthetized by inhalation of an isoflurane/air mixture (2%, 1 L/min) before intramuscular injection of 1×106 mouse MOS-J osteosarcoma cells in close proximity to the tibia, leading to a rapidly growing tumor in soft tissue with secondary contiguous bone invasion. Tumors appeare at the injection site 8 days later and lead to osteoblastic lesions reproducing the osteoblastic form of human osteosarcoma. Three groups (n=6 per group) of C57Bl/6J are assigned randomly to receive either placebo or Alpelisib (BYL719) (oral administration, 12.5-50 mg/kg daily). The preventive treatment starts 1 day after tumor cells inoculation. Four groups of 6 C57Bl/6J are assigned randomly to receive either placebo (oral administration of methylcellulose 0.5% and intraperitoneal injection of water), Alpelisib (BYL719) (oral administration of 50 mg/kg per day), ifosfamide (intraperitoneal injection of 30 mg/kg three times during the first week), or a combination of Alpelisib (BYL719) (50 mg/kg daily) and ifosfamide (30 mg/kg, three times during the first week). |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C19H22F3N5O2S |
| Molecular Weight | 441.470 |
| Exact Mass | 441.144623 |
| PSA | 133.93000 |
| LogP | -0.02 |
| Index of Refraction | 1.587 |
| Storage condition | -20℃ |
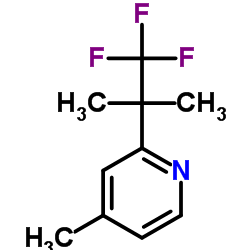
| Precursor 6 | |
|---|---|
| DownStream 0 | |