145435-72-9
| Name | gamithromycin |
|---|---|
| Synonyms |
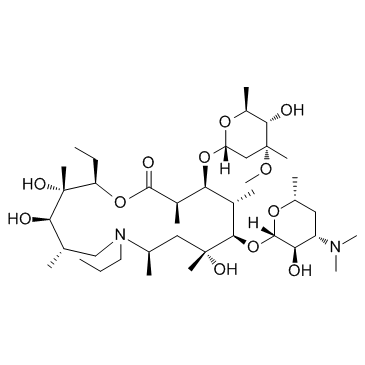
(2R,3S,4R,5S,8R,10R,11R,12S,13S,14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,8,10,12,14-hexamethyl-7-propyl-11-{[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy}-1-oxa-7-azacylopentadecan-15-one
Zactran (2R,3S,4R,5S,8R,10R,11R,12S,13S,14R)-2-Ethyl-3,4,10-trihydroxy-3,5,8,10,12,14-hexamethyl-15-oxo-7-propyl-11-{[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy}-1-oxa-7-azacyclopentadecan-13-yl 2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranoside 1-Oxa-7-azacyclopentadecan-15-one, 13-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,8,10,12,14-hexamethyl-7-propyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]-, (2R,3S,4R,5S,8R,10R,11R,12S,13S,14R)- Gamithromycin |
| Description | Gamithromycin is an antimicrobial agent which can inhibit the growth of MmmSC strains B237 and Tan8 with MICs of 0.00012 and 0.00006 μg/mL, respectively. |
|---|---|
| Related Catalog | |
| Target |
MIC: 0.00012 μg/mL (MmmSC strain B237), 0.00006μg/mL (MmmSC strain Tan8)[1] |
| In Vitro | The MIC values in serum are significantly lower than those in artificial medium; at an initial inoculum size of 106 cfu/mL, these are 64-, 8- and 64-fold lower for gamithromycin, tylosin and tilmicosin, respectively, against MmmSC strain B237 in serum compare to artificial medium. A similar pattern emerges for Tan8. Heat-inactivation of serum results in an MIC for gamithromycin that is higher than in either non-treated serum or artificial medium[1]. |
| In Vivo | The proportion of foals that recover without the need for a change in treatment is significantly (P<0.048) higher for foals treated with Gamithromycin (GAM) (38 of 40; 95%) or AZM-RIF (39 of 40; 98%) compare to control foals (32 of 41; 78%). The clinical scores, number of abscesses and the abscess scores after 1 and 2 weeks of treatment are significantly lower for foals treated with Gamithromycin (GAM) or AZM-RIF compare to control foals. The WBC count of foals treated with Gamithromycin (GAM) is significantly higher than that of foals treated with AZM-RIF on week 3 of treatment[2]. |
| Cell Assay | Minimum inhibitory concentrations (MICs) for gamithromycin, tylosin and tilmicosin against MmmSC strains B237 and Tan8 are determined using a macrodilution technique. Equal volumes of MmmSC culture in logarithmic phase are added to each antimicrobial dilution to give an inoculum size of 107 cfu/mL, i.e. the intending initial titre for subsequent time-kill assays, in a volume of 4 mL. Cultures are incubated for 24 h at 37°C. At 0 and 24 h time points, samples are removed and serially diluted 10-fold down to 10-5. Aliquots (10 μL) of each dilution are transferred to solid medium; after incubation at 37°C in a humidified atmosphere of 5% carbon dioxide in air for at least 4 days, colonies are counted from the dilution that yields between 30 and 300 colonies per plate. Counts are converted into cfu/mL and MIC is defined as the lowest concentration of antimicrobial that prevents an increase in cfu/mL over 24 h[1]. |
| Animal Admin | Foals with ultrasonographic evidence of pulmonary abscesses are randomly assigned in 3 treatment groups: (1)gamithromycin at a dose of 6.0 mg/kg body weight is administered in the semimembranosus/semitendinosus muscles once a week (GAM; n=40); (2) azithromycin at a dose of 10 mg/kg PO once daily in combination with rifampin at a dose of 10 mg/kg PO once daily (AZM-RIF; n=40); and (3) no antimicrobial treatment (controls; n=41). All the foals in each treatment group also receive acetylcysteine at a dose of 10 mg/kg PO a day to provide the same daily manipulation of the foals in each group[2]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 833.0±65.0 °C at 760 mmHg |
| Molecular Formula | C40H76N2O12 |
| Molecular Weight | 777.038 |
| Flash Point | 457.6±34.3 °C |
| Exact Mass | 776.539856 |
| PSA | 180.08000 |
| LogP | 3.89 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.535 |
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H361d |
| Precautionary Statements | P281-P305 + P351 + P338 |
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |