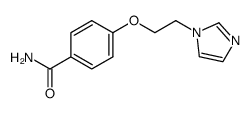
74226-22-5
| Name | 4-(2-imidazol-1-ylethoxy)benzoic acid,hydrochloride |
|---|---|
| Synonyms |
Dazoxiben hydrochloride
Dazoxiben HCl |
| Description | Dazoxiben is a potent and orally active thromboxane (TX) synthase inhibitor[1]. |
|---|---|
| Related Catalog | |
| Target |
Thromboxane (TX) synthase[1] |
| In Vitro | Dazoxiben inhibits TXB2 production in clotting human whole blood with an IC50 of 0.3 μM and causes parallel enhancement of PGE2 greater than PGF2 alpha greater than 6-keto-PGF1 alpha production[1]. Dazoxiben inhibits TXB2 production in rat kidney glomeruli with an IC50 of 1.60 μM) than in rat whole blood (IC50= 0.32μM), and is not associated with changes in PGE2, PGF2 alpha and 6-keto-PGF1 alpha production[1]. |
| In Vivo | Dazoxiben (intraperitoneal administration; 100 μg/kg) produces a marked prolongation of the tail bleeding time with 96.8 ± 10.8 secs[2]. |
| References |
| Boiling Point | 491.2ºC at 760 mmHg |
|---|---|
| Molecular Formula | C12H13ClN2O3 |
| Molecular Weight | 268.69600 |
| Flash Point | 250.9ºC |
| Exact Mass | 268.06100 |
| PSA | 64.35000 |
| LogP | 2.46230 |
| Hazard Codes | Xi |
|---|
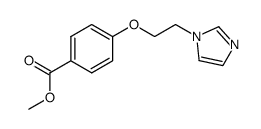
|
~79% 
74226-22-5 |
| Literature: Ono Pharmaceutical Co., Ltd. Patent: US4461905 A1, 1984 ; |
|
~84% 
74226-22-5 |
| Literature: Cross, Peter E.; Dickinson, Roger P.; Parry, M. John; Randall, Michael J. Journal of Medicinal Chemistry, 1985 , vol. 28, # 10 p. 1427 - 1432 |
|
~% 
74226-22-5 |
| Literature: Cross, Peter E.; Dickinson, Roger P.; Parry, M. John; Randall, Michael J. Journal of Medicinal Chemistry, 1985 , vol. 28, # 10 p. 1427 - 1432 |
|
~% 
74226-22-5 |
| Literature: Biagi; Dell'Omodarme; Ferretti; Giorgi; Livi; Scartoni Farmaco, 1990 , vol. 45, # 11 p. 1181 - 1192 |