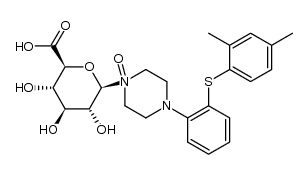
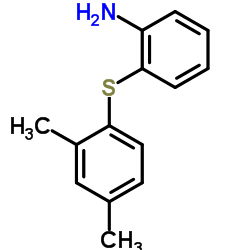
960203-27-4
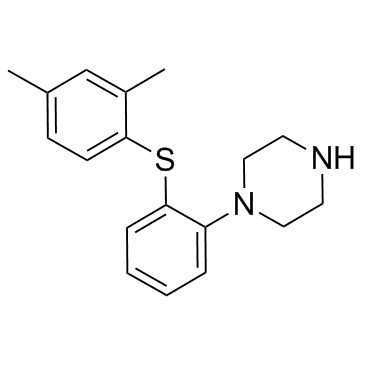
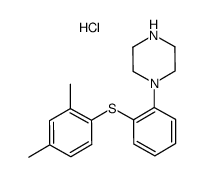
| Name | vortioxetine hydrobromide |
|---|---|
| Synonyms |
Brintellix
1-{2-[(2,4-Dimethylphenyl)sulfanyl]phenyl}piperazine hydrobromide (1:1) Piperazine, 1-[2-[(2,4-dimethylphenyl)thio]phenyl]-, hydrobromide (1:1) UNII:TKS641KOAY VORTIOXETINE HBr Vortioxetine hydrobromide vortioxetine monohydrobromide 1-(2-((2,4-Dimethylphenyl)sulfanyl)phenyl)piperazine monohydrobromide 1-[2-[(2,4-Dimethylphenyl)thio]phenyl]piperazinehydrobromide 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine hydrobromide Vortioxetine (hydrobromide) |
| Description | Vortioxetine hydrobromide is a multimodal serotonergic agent, inhibits 5-HT1A, 5-HT1B, 5-HT3A, 5-HT7 receptor and SERT with Ki values of 15 nM, 33 nM, 3.7 nM, 19 nM and 1.6 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
5-HT1A Receptor:15 nM (Ki) 5-HT1B Receptor:33 nM (Ki) 5-HT3A Receptor:3.7 nM (Ki) 5-HT7 Receptor:19 nM (Ki) SERT:1.6 nM (Ki) |
| In Vitro | Vortioxetine (Compound 5m) is a multimodal serotonergic agent, inhibits 5-HT1A, 5-HT1B, 5-HT3A, 5-HT7 receptor and SERT with Ki values of 15 nM, 33 nM, 3.7 nM, 19 nM and 1.6 nM, respectively. Vortioxetine displays antagonistic properties at 5-HT3A and 5-HT7 receptors, partial agonist properties at 5-HT1B receptors, agonistic properties at 5-HT1A receptors, and potent inhibition of SERT[1]. Vortioxetine is a partial h5-HT 1B receptor agonist with EC50 of 460 nM and intrinsic activity of 22% using a whole-cell cAMP-based assay. Vortioxetine binds to the r5-HT 7 receptor with a Ki value of 200 nM and is a functional antagonist at the r5-HT7 receptor with an IC50 of 2 μM in an in vitro whole-cell cAMP assay[5]. |
| In Vivo | Vortioxetine (Lu AA21004) occupies the r5-HT1B receptor and rSERT (ED50= 3.2 and 0.4 mg/kg, respectively) after subcutaneous administration and is a 5-HT3 receptor antagonist[6]. Vortioxetine significantly increases cell proliferation and cell survival and stimulates maturation of immature granule cells in the sub granular zone of the dentate gyrus of the hippocampus after 21 days of treatment[3]. Vortioxetine does not cause cognitive or psychomotor impairment[4]. |
| References |
| Molecular Formula | C18H23BrN2S |
|---|---|
| Molecular Weight | 379.358 |
| Exact Mass | 378.076538 |
| PSA | 40.57000 |
| LogP | 5.21610 |
| Storage condition | -20°C |
|
~84% 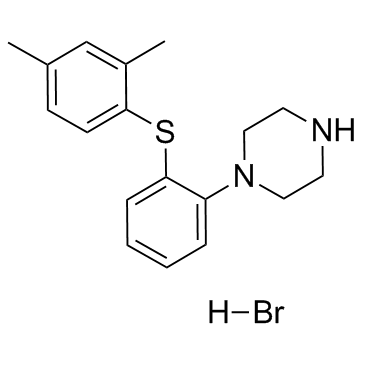
960203-27-4 |
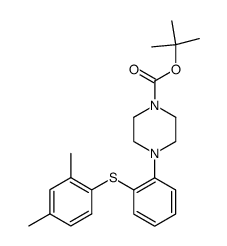
| Literature: H. LUNDBECK A/S; CHRISTENSEN, Kim, Lasse Patent: WO2013/102573 A1, 2013 ; Location in patent: Page/Page column 13; 14 ; |
|
~81% 
960203-27-4 |
| Literature: H. LUNDBECK A/S Patent: WO2008/113359 A2, 2008 ; Location in patent: Page/Page column 16 ; WO 2008/113359 A2 |
|
~% 
960203-27-4 |
| Literature: H. LUNDBECK A/S Patent: WO2008/113359 A2, 2008 ; Location in patent: Page/Page column 17 ; WO 2008/113359 A2 |
|
~83% 
960203-27-4 |
| Literature: H. LUNDBECK A/S; CHRISTENSEN, Kim, Lasse Patent: WO2013/102573 A1, 2013 ; Location in patent: Page/Page column 14; 15 ; |
|
~% 
960203-27-4 |
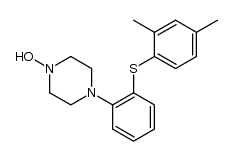
| Literature: H. LUNDBECK A/S Patent: WO2007/144005 A1, 2007 ; Location in patent: Page/Page column 44-45 ; WO 2007/144005 A1 |
|
~% 
960203-27-4 |
| Literature: H. LUNDBECK A/S Patent: WO2008/113359 A2, 2008 ; Location in patent: Page/Page column 18 ; WO 2008/113359 A2 |
|
~% 
960203-27-4 |
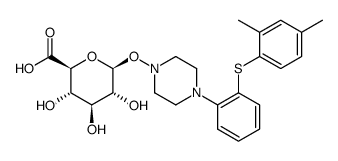
| Literature: Uldam, Henriette Kold; Juhl, Martin; Pedersen, Henrik; Dalgaard, Lars Drug Metabolism and Disposition, 2011 , vol. 39, # 12 p. 2264 - 2274 |
|
~% 
960203-27-4 |
| Literature: Uldam, Henriette Kold; Juhl, Martin; Pedersen, Henrik; Dalgaard, Lars Drug Metabolism and Disposition, 2011 , vol. 39, # 12 p. 2264 - 2274 |
|
~% 
960203-27-4 |
| Literature: Uldam, Henriette Kold; Juhl, Martin; Pedersen, Henrik; Dalgaard, Lars Drug Metabolism and Disposition, 2011 , vol. 39, # 12 p. 2264 - 2274 |