5534-09-8
| Name | beclomethasone dipropionate |
|---|---|
| Synonyms |
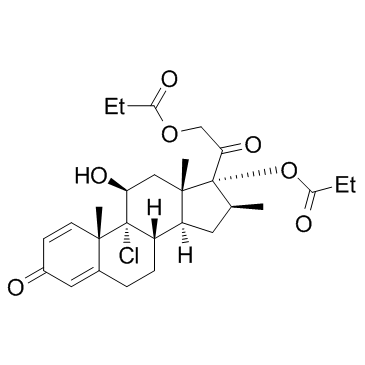
9-Chloro-16-β-methyl-11-β,17,21-trihydroxypregna-1,4-diene-3,20-dione 17,21-Dipropionate
Viarex Beconasol Becloval (11b,16b)-9-Chloro-11-hydroxy-16-methyl-17,21-bis(1-oxopropoxy)-pregna-1,4-diene-3,20-dione (8S,9R,10S,11S,13S,14S,16S,17R)-9-chloro-11-hydroxy-10,13,16-trimethyl-3-oxo-17-[(propanoyloxy)acetyl]-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl propanoate Becodisks pregna-1,4-diene-3,20-dione, 9-chloro-11-hydroxy-16-methyl-17,21-bis(1-oxopropoxy)-, (11b,16b)- Anceron Vancenase AQ propanoate de (8S,9R,10S,11S,13S,14S,16S,17R)-9-chloro-11-hydroxy-10,13,16-triméthyl-3-oxo-17-[(propanoyloxy)acétyl]-6,7,8,9,10,11,12,13,14,15,16,17-dodécahydro-3H-cyclopenta[a]phénanthrén-17-yle (11b,16b)-9-chloro-11-hydroxy-16-methyl-3,20-dioxopregna-1,4-diene-17,21-diyl dipropanoate Pregna-1,4-diene-3,20-dione, 9-chloro-11-hydroxy-16-methyl-17,21-bis(1-oxopropoxy)-, (11β,16β)- (11β,16β)-9-Chloro-11-hydroxy-16-methyl-3,20-dioxopregna-1,4-diene-17,21-diyl dipropanoate EINECS 226-886-0 Beclomethasone dipropionate (USP) Beclomethasone dipropionate Andion MFCD00135613 (8S,9R,10S,11S,13S,14S,16S,17R)-9-Chlor-11-hydroxy-10,13,16-trimethyl-3-oxo-17-[(propanoyloxy)acetyl]-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-ylpropanoat 9-Chloro-11b-hydroxy-16b-methylpregna-1,4-diene-3,20-dione 17,21-Dipropionate Beclometasone dipropionate |
| Description | Beclometasone dipropionate is a potent glucocorticoid agonist; it is a prodrug of the free form, beclometasone. IC50 Value: 0.2 nM (Inhibiting thymidine incorporation) [1]Target: glucocorticoid receptorin vitro: Cortisol and beclomethasone dipropionate were more potent than salbutamol in inhibiting thymidine incorporation with IC50 values of 5 nM and 0.2 nM respectively. Cortisol 100 nM led to a 16.6 +/- 6.5% reduction and beclomethasone dipropionate 3 nM led to a 17.8 +/- 5.8% reduction in cell number [1]. in vivo: Controlled trials involving 497 adults and children demonstrated similar clinical efficacy between nebulized BDP and either nebulized fluticasone propionate or nebulized budesonide. Meta-analyses show that BDP, like other inhaled corticosteroids, has no major influence on patient height, urinary cortisol concentration, or bone metabolism [2]. Beclometasone dipropionate (BDP) 800 microgday(-1) suspension for nebulization and budesonide (BUD) 750 microg day(-1) given by nebulization in a twice-daily regimen, and when used in addition to the usual maintenance therapy, resulted in comparable clinical efficacy across all parameters [3]. Clinical trial: Efficacy and Tolerability of Beclometasone/Formoterol Single Inhaler in Patients With Moderate to Severe Persistent Asthma. Phase 3 |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 630.5±55.0 °C at 760 mmHg |
| Melting Point | 210ºC |
| Molecular Formula | C28H37ClO7 |
| Molecular Weight | 521.042 |
| Flash Point | 335.1±31.5 °C |
| Exact Mass | 520.222778 |
| PSA | 106.97000 |
| LogP | 4.59 |
| Vapour Pressure | 0.0±4.2 mmHg at 25°C |
| Index of Refraction | 1.564 |
|
Section 1. Chemical Product and Company Identification Beclomethasone Dipropionate Common Name/ Trade Name Beclomethasone Dipropionate Section 3. Hazards Identification Potential Acute Health Effects Slightly hazardous in case of skin contact (irritant), of eye contact (irritant), of ingestion, of inhalation.
Potential Chronic HealthCARCINOGENIC EFFECTS: Not available. EffectsMUTAGENIC EFFECTS: Not available. TERATOGENIC EFFECTS: Classified POSSIBLE for human. DEVELOPMENTAL TOXICITY: Classified Development toxin [POSSIBLE]. Repeated exposure to a highly toxic material may produce general deterioration of health by an accumulation in one or many human organs. Section 4. First Aid Measures Eye ContactCheck for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Cold water may be used. WARM water MUST be used. Get medical attention if irritation occurs. Skin ContactWash with soap and water. Cover the irritated skin with an emollient. Get medical attention if irritation develops. Serious Skin ContactNot available. InhalationIf inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention immediately. Serious InhalationNot available. IngestionDo NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Loosen tight clothing such as a collar, tie, belt or waistband. Get medical attention if symptoms appear. Serious IngestionNot available. Section 5. Fire and Explosion Data Flammability of the Product May be combustible at high temperature. Auto-Ignition Temperature Not available. Flash PointsNot available. Flammable LimitsNot available. Products of CombustionThese products are carbon oxides (CO, CO2), halogenated compounds. Fire Hazards in Presence of Slightly flammable to flammable in presence of heat. Various Substances Explosion Hazards in Presence Risks of explosion of the product in presence of mechanical impact: Not available. of Various SubstancesRisks of explosion of the product in presence of static discharge: Not available. Fire Fighting MediaSMALL FIRE: Use DRY chemical powder. and InstructionsLARGE FIRE: Use water spray, fog or foam. Do not use water jet. Special Remarks onAs with most organic solids, fire is possible at elevated temperatures Fire Hazards Special Remarks on Explosion Fine dust dispersed in air in sufficient concentrations, and in the presence of an ignition source is a potential dust Hazardsexplosion hazard. Beclomethasone Dipropionate Section 6. Accidental Release Measures Small SpillUse appropriate tools to put the spilled solid in a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and dispose of according to local and regional authority requirements. Large SpillUse a shovel to put the material into a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and allow to evacuate through the sanitary system. Section 7. Handling and Storage PrecautionsKeep away from heat. Keep away from sources of ignition. Ground all equipment containing material. Do not breathe dust. StorageKeep container tightly closed. Keep container in a cool, well-ventilated area. It should be stored at 25 deg. C (77 deg. F), but storage at temperatures between 15 and 30 deg. C (59 to 86 deg. F) are acceptable. The product should not be exposed to temperatures above 120 deg. F. because container may burst. Section 8. Exposure Controls/Personal Protection Engineering ControlsUse process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit. Personal ProtectionSafety glasses. Lab coat. Dust respirator. Use a dust respirator is ventilation if inadequate and handling of product creates visible dust clouds. Be sure to use an approved/certified respirator or equivalent. Gloves. Personal Protection in Case of Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used a Large Spillto avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product. Exposure LimitsNot available. Section 9. Physical and Chemical Properties Physical state and appearance Solid. (Solid powder.)OdorOdorless. TasteNot available. 521.1 g/mole Molecular Weight ColorWhite to creamy pH (1% soln/water)Not available. Not available. Boiling Point Melting Point201°C (393.8°F) - 208 C.B^ Not available. Critical Temperature Specific GravityNot available. Vapor PressureNot applicable. Vapor DensityNot available. VolatilityNot available. Odor ThresholdNot available. Water/Oil Dist. Coeff.Not available. Not available. Ionicity (in Water) Dispersion PropertiesNot available. Very slightly soluble in cold water. Solubility Beclomethasone Dipropionate Section 10. Stability and Reactivity Data The product is stable. Stability Instability TemperatureNot available. Excess heat Conditions of Instability Not available. Incompatibility with various substances CorrosivityNot available. Special Remarks onNot available. Reactivity Special Remarks onNot available. Corrosivity PolymerizationWill not occur. Section 11. Toxicological Information Routes of EntryInhalation. Ingestion. Toxicity to AnimalsWARNING: THE LC50 VALUES HEREUNDER ARE ESTIMATED ON THE BASIS OF A 4-HOUR EXPOSURE. Acute oral toxicity (LD50): >3750 mg/kg [Rat]. Acute toxicity of the dust (LC50): 51.6 mg/m3 2 hours [Rat]. Chronic Effects on Humans Not available. Other Toxic Effects onSlightly hazardous in case of skin contact (irritant), of ingestion, of inhalation. Humans Special Remarks on Not available. Toxicity to Animals Special Remarks onNot available. Chronic Effects on Humans Special Remarks on otherAcute Potential Health Effects: Toxic Effects on HumansSkin: May cause skin irritation. Eyes: Dust may cause eye irritation. Inhalation: A massive single dose is unlikely to cause adverse effects. Dust may cause respiratory tract (nasopharyngeal) irritaiton with nasopharyngitis, nasal burning, rhinorrhea, hoarseness, bronchospasm, coughing and possible nasal septum perforation. It may also cause headache, nausea, vomiting. Ingestion: May cause gastrointestinal tract irritation with nausea, vomiting, hypermotility, diarrhea. Other adverse effects of inhalation or ingestion may include: decreased or blurred vision, frequent urination, increased thirst, increased appetite, indegestion, nervousness, convulsions, seizures, muscle contraction or spasticity, trouble sleeping weight gain, dizziness, flushing of the face or cheeks, hiccups, and increased sweating. Chronic Potential Health Effects: Inhalation or Ingestion: Prolonged or repeated inhalation or ingestion may cause possible hypersensitization; acne or other skin problems; hip or shoulder pain; osteoporosis; gradual blurring or loss of vision (cataracts or glaucoma); eye pain or redness; tearing and sensitivity of eyes to light; rounding out of the face; purple lines on arms, face, legs, trunk, groin; unusual increase in hair growth; hypertension; menstrual irregularities; muscle weakness; unusual bruising; rapid weight gain; swelling of feet or lower legs; irregular heartbeat; muscle cramps or pain; unusual tiredness or weakness; impaired wound healing; headache; insomnia; pain in back, ribs, arms or legs; continuing abdominal or stomach pain or burning; nausea; vomiting; bloody or black tarry stools; tendon rupture; thin, fragile skin; blood (changes in erythrocyte and leukocyte count), thymus, and spleen Medical Conditions Aggravated by Exposure: Hypersensitivity to material; ocular herpes simplex; AIDS or HIV infection; heart disease; congestive heart failure; recent heart attack; intestinal anastomoses; strongyloides infestation; diabetes mellitus; mayasthenia gravis; impaired kidney function or disease; esophagitis; gastritis or peptic ulcer; chicken pox or measles (including recent exposure); tuberculosis; system fungal infections. Beclomethasone Dipropionate Section 12. Ecological Information EcotoxicityNot available. BOD5 and CODNot available. Products of BiodegradationPossibly hazardous short term degradation products are not likely. However, long term degradation products may arise. The products of degradation are as toxic as the product itself. Toxicity of the Products of Biodegradation Special Remarks on theNot available. Products of Biodegradation Section 13. Disposal Considerations Waste DisposalWaste must be disposed of in accordance with federal, state and local environmental control regulations. Section 14. Transport Information DOT ClassificationNot a DOT controlled material (United States). Not applicable. Identification Not applicable. Special Provisions for Transport DOT (Pictograms) Section 15. Other Regulatory Information and Pictograms No products were found. Federal and State Regulations CaliforniaCalifornia prop. 65: This product contains the following ingredients for which the State of California has found to cause cancer which would require a warning under the statute: No products were found. Proposition 65 Warnings California prop. 65: This product contains the following ingredients for which the State of California has found to cause birth defects which would require a warning under the statute: Beclomethasone Dipropionate Other RegulationsEINECS: This product is on the European Inventory of Existing Commercial Chemical Substances. EINECS no. 226-886-0. WHMIS (Canada) Not controlled under WHMIS (Canada). Other Classifications DSCL (EEC)This product is not classified according Not applicable. to the EU regulations. Health Hazard HMIS (U.S.A.)1 National Fire Protection 1 Flammability 1 Association (U.S.A.) Fire Hazard 1 0 Reactivity Health Reactivity 0 Specific hazard Personal Protection E Beclomethasone Dipropionate WHMIS (Canada) (Pictograms) DSCL (Europe) (Pictograms) TDG (Canada) (Pictograms) ADR (Europe) (Pictograms) Protective Equipment Gloves. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Beclomethasone Dipropionate All chemicals may pose unknown hazards and should be used with caution. This Material Safety Data Sheet (MSDS) applies only to the material as packaged. If this product is combined with other materials, deteriorates, or becomes contaminated, it may pose hazards not mentioned in this MSDS. It shall be the user's responsibility to develop proper methods of handling and personal protection based on the actual conditions of use. While this MSDS is based on technical data judged to be reliable, Spectrum Quality Products, SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H360 |
| Precautionary Statements | P201-P308 + P313 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R60;R61 |
| Safety Phrases | S53-S36/37/39-S45 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | TU3805000 |
| HS Code | 2937290090 |
| HS Code | 2937290090 |
|---|