2174-59-6
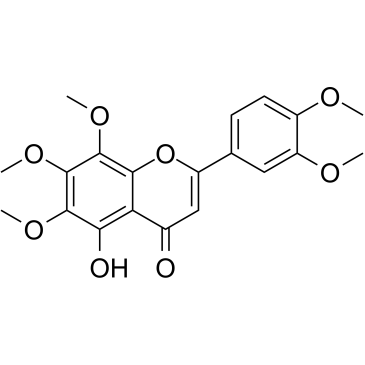
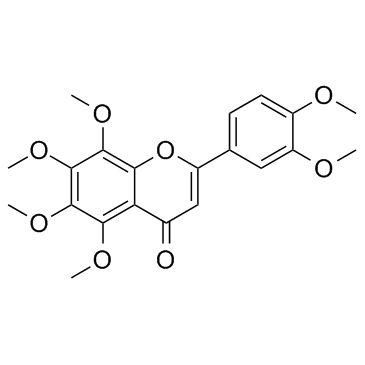
| Name | 2-(3,4-dimethoxyphenyl)-5-hydroxy-6,7,8-trimethoxychromen-4-one |
|---|---|
| Synonyms |
4H-1-Benzopyran-4-one, 2-(3,4-dimethoxyphenyl)-5-hydroxy-6,7,8-trimethoxy-
2-(3,4-Dimethoxyphenyl)-5-hydroxy-6,7,8-trimethoxy-4H-chromen-4-one 5-hydroxy-6,7,8,3',4'-pentamethoxyflavone Demethylnobiletin 5-O-Demethylnobiletin 5-hydroxy-3',4',6,7,8-pentamethoxyflavone 5-Demethylnobiletin 5-desmethylnobeletin 5-desmethylnobiletin |
| Description | 5-O-Demethylnobiletin (5-Demethylnobiletin), a polymethoxyflavone isolated from Sideritis tragoriganum, is a direct inhibition of 5-LOX (IC50=0.1 μM), without affecting the expression of COX-2. 5-O-Demethylnobiletin (5-Demethylnobiletin) has anti-inflammatory activity, inhibits leukotriene B (4)(LTB4) formation in rat neutrophils and elastase release in human neutrophils with an IC50 of 0.35 μM[1].5-O-Demethylnobiletin (5-demethylnobiletin) promotes neuritogenesis through the activation of MAPK/ERK-, PKC-, and PKA-dependent signaling pathways[2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.1 μM (Lox 5); 0.35 μM (LTB4) (5-O-Demethylnobiletin)[1] |
| References |
| Density | 1.304±0.06 g/cm3 |
|---|---|
| Boiling Point | 601.4±55.0 °C at 760 mmHg |
| Melting Point | 145-146 ºC |
| Molecular Formula | C20H20O8 |
| Molecular Weight | 388.368 |
| Flash Point | 213.9±25.0 °C |
| Exact Mass | 388.115814 |
| PSA | 96.59000 |
| LogP | 2.10 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.583 |
| Storage condition | 2-8C |
| Water Solubility | Practically insoluble (0.058 g/L) (25 ºC) |
| HS Code | 2914509090 |
|---|
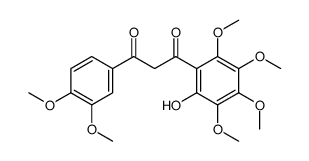
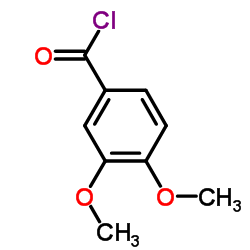
| Precursor 10 | |
|---|---|
| DownStream 1 | |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |

![(1H-benzo[d][1,2,3]triazol-1-yl)(3,4-dimethoxyphenyl)methanone structure](https://image.chemsrc.com/caspic/248/333347-81-2.png)