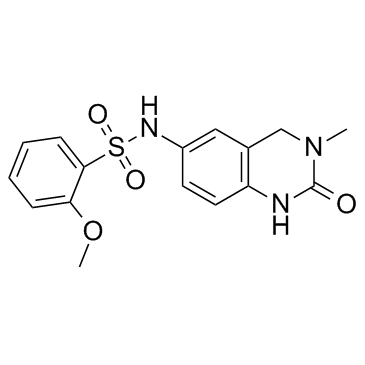
1403764-72-6
| Name | 2-methoxy-N-(3-methyl-2-oxo-1,4-dihydroquinazolin-6-yl)benzenesulfonamide |
|---|---|
| Synonyms |
2-Methoxy-N-(3-methyl-2-oxo-1,2,3,4-tetrahydro-6-quinazolinyl)benzenesulfonamide
Benzenesulfonamide, 2-methoxy-N-(1,2,3,4-tetrahydro-3-methyl-2-oxo-6-quinazolinyl)- QCR-192 PFI-1 CS-1362 PF-6405761 |
| Description | PFI-1 is a selective BET (bromodomain-containing protein) inhibitor for BRD4 with IC50 of 0.22 μM in a cell-free assay. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.22 μM (BRD4) |
| In Vitro | PFI-1 has antiproliferative effects on leukemic cell lines and efficiently abrogates their clonogenic growth. Exposure of sensitive cell lines with PFI-1 results in G1 cell-cycle arrest, downregulation of MYC expression, as well as induction of apoptosis and induces differentiation of primary leukemic blasts. Cells exposed to PFI-1 show significant downregulation of Aurora B kinase, thus attenuating phosphorylation of the Aurora substrate H3S10, providing an alternative strategy for the specific inhibition of this well-established oncology target[1]. PFI-1 binds to with cyclic AMP response binding protein with Kd of 49 μM. PFI-1 has an EC50 of 1.89 μM for the inhibition of IL6 production from human blood mononuclear cells stimulated by LPS[2]. PFI-1 induces dose-dependent reduction of cell viability in T4302 CD133+ cells[3]. PFI-1 inhibits the proliferating of three NET cell lines (Bon-1 derived from a pancreatic NET, and H727 and H720 derived from lung NETs)[4]. |
| In Vivo | PFI-1 administrated (1 mg/kg, i.v.) in the rat results in the volume of distribution of 1 L/kg, the plasma clearance of 18 mL/min/kg and half-life of 1 hour. PFI-1 oral dosed (2 mg/kg) in the rat results in the oral bioavailability as low as 32%. PFI-1 administrated (2 mg/kg, s.c.) in the mouse results in a Cmax of 58 ng/mL with a Tmax of 1 h and a half-life of approximately 2 hours[2]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C16H17N3O4S |
| Molecular Weight | 347.389 |
| Exact Mass | 347.093964 |
| PSA | 96.12000 |
| LogP | 0.53 |
| Appearance | white to beige |
| Index of Refraction | 1.628 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: ≥10mg/mL |
| RIDADR | NONH for all modes of transport |
|---|