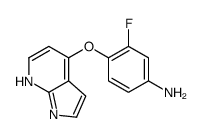
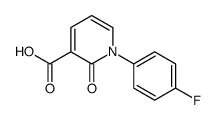
888719-03-7
| Name | 1-(4-fluorophenyl)-N-[3-fluoro-4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)phenyl]-2-oxopyridine-3-carboxamide |
|---|---|
| Synonyms |
1-(4-Fluorophenyl)-N-[3-fluoro-4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)phenyl]-2-oxo-1,2-dihydro-3-pyridinecarboxamide
RS0065 3ce3 2-pyridone analogue,2 N-(4-(1H-Pyrrolo[2,3-b]pyridin-4-yloxy)-3-fluorophenyl)-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide 3-Pyridinecarboxamide, 1-(4-fluorophenyl)-N-[3-fluoro-4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)phenyl]-1,2-dihydro-2-oxo- |
| Description | MET kinase-IN-4 is an orally active Met kinase inhibitor. MET kinase-IN-4 has potent Met kinase inhibitory activity with an IC50 value of 1.9 nM. MET kinase-IN-4 can be used for the research of cancer[1]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 1.9 nM (Met); 4 nM (Flt-3); 27 nM (VEGFR-2)[1] |
| In Vitro | MET kinase-IN-4 (Compound 2) 具有有效的 Met 激酶抑制活性,IC50 值为 1.9 nM[1]。 MET kinase-IN-4 抑制 Flt-3 和 VEGFR-2 激酶,IC50 值分别为 4 和 27 nM[1]。 MET kinase-IN-4 (3 μM) 在人和小鼠肝微粒体中表现出良好的代谢稳定性[1]。 |
| In Vivo | MET kinase-IN-4 (Compound 2) 在小鼠体内具有良好的药代动力学特征[1]。 MET kinase-IN-4 在 GTL-16 人胃癌异种移植模型中表现出显著的体内抗肿瘤活性[1]。 Animal Model: Mice[1] Dosage: 5, 10 mg/kg Administration: IV, PO Result: Showed extensive extravascular distribution and a favorable half-life. Animal Model: Nude mice[1] Dosage: 6.25, 12.5, 25 and 50 mg/kg Administration: PO, once a day Result: Showed antitumor activity in dose-dependet. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 701.5ºC at 760 mmHg |
| Melting Point | 212-214ºC |
| Molecular Formula | C25H16F2N4O3 |
| Molecular Weight | 458.416 |
| Flash Point | 378.1ºC |
| Exact Mass | 458.119049 |
| PSA | 89.01000 |
| LogP | 4.82 |
| Index of Refraction | 1.721 |
|
~78% 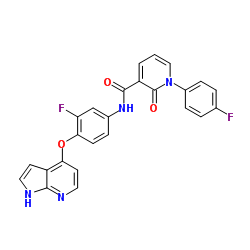
888719-03-7 |
| Literature: Kyoung, Soon Kim; Zhang, Liping; Schmidt, Robert; Cai, Zhen-Wei; Wei, Donna; Williams, David K.; Lombardo, Louis J.; Trainor, George L.; Xie, Dianlin; Zhang, Yaquan; An, Yongmi; Sack, John S.; Tokarski, John S.; Darienzo, Celia; Kamath, Amrita; Marathe, Punit; Zhang, Yueping; Lippy, Jonathan; Jeyaseelan Sr., Robert; Wautlet, Barri; Henley, Benjamin; Gullo-Brown, Johnni; Manne, Veeraswamy; Hunt, John T.; Fargnoli, Joseph; Borzilleri, Robert M. Journal of Medicinal Chemistry, 2008 , vol. 51, # 17 p. 5330 - 5341 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |