850879-09-3
| Name | N-(1,3-benzodioxol-5-ylmethyl)-4-([1]benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide |
|---|---|
| Synonyms |
Amuvatinib (MP-470)
MP470,MP-470 Amuvatinib 4-benzo[4,5]furo[3,2-d]pyrimidin-4-yl-piperazine-1-carbothioic acid (benzo[1,3]dioxol-5-ylmethyl)-amide N-(1,3-Benzodioxol-5-ylmethyl)-4-benzofuro[3,2-d]pyrimidin-4-yl-1-piperazinecarbothioamide Amuvatinib (MP-470,HPK 56) N-(benzo[d][1,3]dioxol-5-ylmethyl)-4-(benzofuro[3,2-d]pyrimidin-4-yl)piperazine-1-carbothioamide HPK56 MP 470 S1244_Selleck N-(1,3-Benzodioxol-5-ylmethyl)-4-([1]benzofuro[3,2-d]pyrimidin-4-yl)-1-piperazinecarbothioamide 1-Piperazinecarbothioamide, N-(1,3-benzodioxol-5-ylmethyl)-4-(benzofuro[3,2-d]pyrimidin-4-yl)- |
| Description | Amuvatinib (MP-470) is a potent and multi-targeted inhibitor of c-Kit, PDGFRα and Flt3 with IC50 of 10 nM, 40 nM and 81 nM, respectively.IC50 Value: 10 nM(c-KitD816H); 40 nM(PDGFRαV561D); 81 nM(Flt3D835Y) [1]Target: c-Kit; PDGFRα; FLT3in vitro: The hydrochloride salt of MP-470 also inhibits several mutants of c-Kit, including c-KitD816V, c-KitD816H, c-KitV560G, and c-KitV654A, as well as a Flt3 mutant (Flt3D835Y) and two PDGFRα mutants (PDGFRαV561D and PDGFRαD842V), with IC50 of 10 nM to 8.4 μM. MP-470 potently inhibits the proliferation of OVCAR-3, A549, NCI-H647, DMS-153, and DMS-114 cells, with IC50 of 0.9 μM–7.86 μM [1]. MP-470 also inhibits c-Kit and PDGFRα, with IC50 values of 31 μM and 27 μM, respectively. MP-470 demonstrates potent cytotoxicity against MiaPaCa-2, PANC-1, and GIST882 cells, with IC50 of 1.6 μM to 3.0 μM. MP-470 also binds to and inhibits several c-Kit mutants, including c-KitK642E, c-KitD816V, and c-KitK642E/D816V [2]. In MDA-MB-231 cells, MP-470 (1 μM) inhibits tyrosine phosphorylation of AXL [3]. In LNCaP and PC-3, but not DU145 cells, MP-470 exhibits cytotoxicity with IC50 of 4 μM and 8 μM, respectively, and induces apoptosis at 10 μM. In LNCaP cells, MP-470 (10 μM) elicits G1 arrest and decreases phosphorylation of Akt and ERK1/2 [4].in vivo: In mice xenograft models of HT-29, A549, and SB-CL2 cells, MP-470 (10 mg/kg–75 mg/kg via i.p. or 50 mg/kg–200 mg/kg via p.o.) inhibits tumor growth [1]. In mice bearing LNCaP xenograft, MP-470 (20 mg/kg) combined with Erlotinib significantly induces tumor growth inhibition (TGI) [4]. |
|---|---|
| Related Catalog | |
| References |
[1]. Bearss DJ, et al. US Patent, US/2008/0226747. |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 649.5±65.0 °C at 760 mmHg |
| Molecular Formula | C23H21N5O3S |
| Molecular Weight | 447.509 |
| Flash Point | 346.6±34.3 °C |
| Exact Mass | 447.136505 |
| PSA | 115.02000 |
| LogP | 2.79 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.739 |
| Storage condition | -20°C |
|
~37% 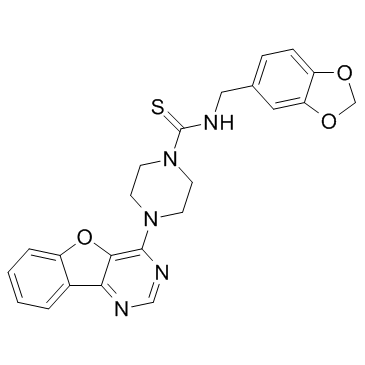
850879-09-3 |
| Literature: ARIZONA BOARD OF REGENTS on behalf of THE UNIVERSITY OF ARIZONA; MONTIGEN PHARMACEUTICALS, INC. Patent: WO2005/37825 A2, 2005 ; Location in patent: Page/Page column 62-63 ; WO 2005/037825 A2 |
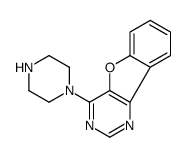
| Precursor 1 | |
|---|---|
| DownStream 0 | |