199739-10-1
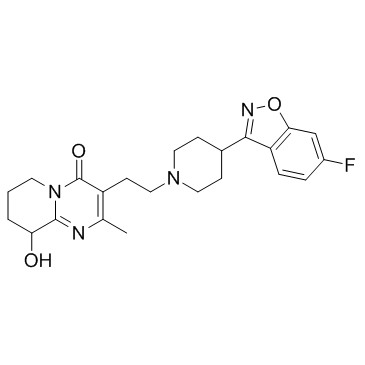
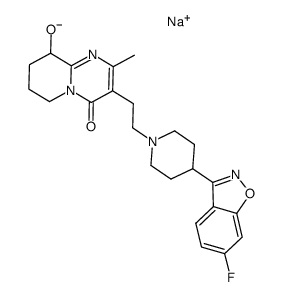
| Name | [3-[2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl]-2-methyl-4-oxo-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-9-yl] hexadecanoate |
|---|---|
| Synonyms |
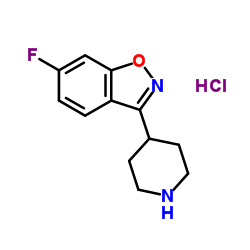
3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido-[1,2-a]pyrimidin-4-one palmitate ester
9-Hydroxyrisperidone palmitate Xeplion PALPERIDONE PALMITATE UNII-R8P8USM8FR Paliperidone Palmitate Invega Sustenna Paliperidone Palmitate [USAN] 3-[2-[4-(6-fluoro-1,2-benzoisoxazol-3-yl)-1-piperidyl]ethyl]-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one palmitate ester Hexadecanoic acid, 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-methyl-4-oxo-4H-pyrido[1,2-a]pyrimidin-9-yl ester 3-{2-[4-(6-Fluoro-1,2-benzoxazol-3-yl)-1-piperidinyl]ethyl}-2-methyl-4-oxo-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-9-yl palmitate |
| Description | Paliperidone palmitate (9-Hydroxyrisperidone palmitate), an atypical long-acting antipsychotic agent, is an ester prodrug of Paliperidone. Paliperidone is a dopamine antagonist and 5-HT2A antagonist of the atypical antipsychotic class. Paliperidone palmitate shows efficacy against schizophrenia[1]. |
|---|---|
| Related Catalog | |
| Target |
5-HT2A Receptor D2 Receptor |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 736.3±70.0 °C at 760 mmHg |
| Molecular Formula | C39H57FN4O4 |
| Molecular Weight | 664.893 |
| Flash Point | 399.1±35.7 °C |
| Exact Mass | 664.436401 |
| PSA | 90.46000 |
| LogP | 9.85 |
| Vapour Pressure | 0.0±2.4 mmHg at 25°C |
| Index of Refraction | 1.590 |
| Storage condition | -20℃ |
|
~77% 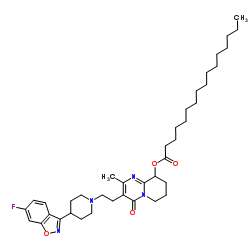
199739-10-1 |
| Literature: WO2012/164582 A1, ; Page/Page column 11-12 ; |
|
~90% 
199739-10-1 |
| Literature: WO2009/89076 A2, ; Page/Page column 15; 29 ; |
|
~81% 
199739-10-1 |
| Literature: WO2009/89076 A2, ; Page/Page column 28 ; |
|
~55% 
199739-10-1 |
| Literature: WO2009/89076 A2, ; Page/Page column 25-26 ; |
|
~10% 
199739-10-1 |
| Literature: WO2009/89076 A2, ; Page/Page column 9; 26 ; |
|
~% 
199739-10-1 |
| Literature: WO2012/164582 A1, ; |
|
~% 
199739-10-1 |
| Literature: WO2012/164582 A1, ; |
| Precursor 6 | |
|---|---|
| DownStream 1 | |