519055-62-0
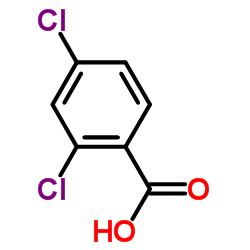
| Name | N-(5-bromothiophen-2-yl)sulfonyl-2,4-dichlorobenzamide |
|---|---|
| Synonyms |
N-[(5-Bromo-2-thienyl)sulfonyl]-2,4-dichlorobenzamide
Benzamide, N-[(5-bromo-2-thienyl)sulfonyl]-2,4-dichloro- 5-Bromo-N-(2,4-dichlorobenzoyl)-2-thiophenesulfonamide Tasisulam cc-591 UNII-1YC4W9MSLJ T5SJ BSWMVR BG DG&& EE LY573636 |
| Description | Tasisulam is a small molecule antitumor agent that inhibits mitotic progression and induces vascular normalization. Tasisulam induces apoptosis via the intrinsic pathway[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Tasisulam (200 μM-200 nM; 48 hours) induces an antiproliferative response across a wide range of tumor histologies with EC50s is 10 μM and 25 μM for Calu-6 and A-375 cell lines, respectively[1]. Tasisulam (25, 50 μM; 72 hours) induces a concentration-dependent increase in 4N DNA and G2-M accumulation[1]. Tasisulam (200 μM-200 nM; 48 hours) induces apoptosis in a broad range of in vitro cancer cell models[1]. Cell Proliferation Assay[1] Cell Line: Calu-6 non-small cell lung carcinoma and A-375 melanoma models Concentration: 200 μM-200 nM Incubation Time: 48 hours Result: Induced an antiproliferative response across a wide range of tumor histologies with EC50s are 10 μM and 25 μM, respectively. Cell Cycle Analysis[1] Cell Line: Calu-6 and A-375 cell lines Concentration: 25, 50 μM Incubation Time: 72 hours Result: Induced a concentration-dependent increase in 4N DNA and G2-M accumulation. Apoptosis Analysis[1] Cell Line: Calu-6 non-small cell lung carcinoma and A-375 melanoma models Concentration: 200 μM-200 nM Incubation Time: 48 hours Result: Induced apoptosis in a broad range of in vitro cancer cell models. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Molecular Formula | C11H6BrCl2NO3S2 |
| Molecular Weight | 415.110 |
| Exact Mass | 412.834930 |
| PSA | 99.86000 |
| LogP | 3.94 |
| Index of Refraction | 1.657 |
| Storage condition | -20℃ |
| HS Code | 2934999090 |
|---|
|
~99% 
519055-62-0 |
| Literature: Kosal, Andrew D.; Wilson, Erin E.; Ashfeld, Brandon L. Chemistry--A European Journal, 2012 , vol. 18, # 45 p. 14444 - 14453,10 Title/Abstract Full Text Show Details Kosal, Andrew D.; Wilson, Erin E.; Ashfeld, Brandon L. Chemistry - A European Journal, 2012 , vol. 18, # 45 p. 14444 - 14453 |
|
~97% 
519055-62-0 |
| Literature: Yates, Matthew H.; Kallman, Neil J.; Ley, Christopher P.; Wei, Jeffrey N. Organic Process Research and Development, 2009 , vol. 13, # 2 p. 255 - 262 |
|
~% 
519055-62-0 |
| Literature: Mader, Mary M.; Shih, Chuan; Considine, Eileen; De Dios, Alfonso; Grossman, Cora Sue; Hipskind, Philip A.; Lin, Ho-Shen; Lobb, Karen L.; Lopez, Beatriz; Lopez, Jose E.; Cabrejas, Luisa M. Martin; Richett, Michael E.; White, Wesley T.; Cheung, Yiu-Yin; Huang, Zhongping; Reilly, John E.; Dinn, Sean R. Bioorganic and Medicinal Chemistry Letters, 2005 , vol. 15, # 3 p. 617 - 620 |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |