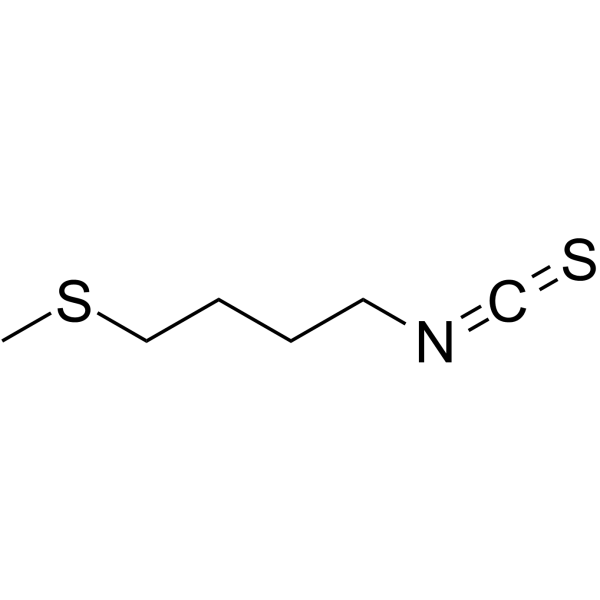
4430-36-8
| Name | 1-isothiocyanato-4-methylsulfanylbutane |
|---|---|
| Synonyms |
4-isothiocyanatobutyl methyl sulfide
4-methylthiobutyl isothiocyanate Butane, 1-isothiocyanato-4-(methylthio)- 1-isothiocyanato-4-methylthiobutane Erucin 1-Isothiocyanato-4-(methylsulfanyl)butane |
| Description | Erucin (ERU) is an isothiocyanate particularly abundant in arugula. Erucin shows anticancer, neuroprotective, and anti-inflammatory activities[1][2][3][4]. |
|---|---|
| Related Catalog | |
| In Vitro | Erucin (ERU) (0-100 μM) releases H2S and inhibits cell viability in AsPC‐1 cells in a concentration-dependent manner[1]. Erucin inhibits cell migration and altered the AsPC‐1 cell cycle, reducing G0/G1 phase and increasing G2/M and S phases[1]. Erucin (30 μM, 72 h) induces AsPC‐1 cell apoptosis and inhibits cell migration[1]. Erucin reduces levels of phosphorylated ERK1/2 in AsPC‐1 cells[1]. Erucin (0-200 μM, 24 h) shows antiproliferative activity with an IC50 of 97.7 µM in A549 cells[2]. Erucin (0-50 μM, 24 h) induces the cleavage of PARP-1 at 50 µM, and increases p53 and p21 protein expression in A549 cells[2]. Erucin decreases LPS-induced production of NO, prostaglandin E2 (PGE2), TNF-α, IL-6 and IL-1β in RAW 264.7 cells[3]. Erucin decreases LPS-induced expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 in RAW 264.7 cells[3]. Erucin inhibits LPS-induced activation of NFκB Signaling in RAW 264.7 cells[3]. Cell Viability Assay[1] Cell Line: AsPC‐1 Concentration: 10, 30, and 100 μM Incubation Time: 72 h Result: Showed a significant and concentration‐dependent reduction of cell viability. Cell Cycle Analysis[1] Cell Line: AsPC‐1 Concentration: 30 μM Incubation Time: 72 h Result: Showed a particular increase of cells number in the G2/M phase (36.6% ± 3.5 vs. vehicle‐treated cells in the G2/M phase: 24.0% ± 1.3) and in the S‐phase (18.1% ± 1.5 vs. vehicle‐treated cells in the S phase: 11.0% ± 0.7) and a consequent significant reduction of cells in the G0/G1 phase (35.1% ± 5.0 vs. vehicle‐treated cells in the G0/G1 phase: 59.5% ± 1.8. Apoptosis Analysis[1] Cell Line: AsPC‐1 Concentration: 30 μM Incubation Time: 72 h Result: Significantly increased the number of total apoptotic cells (apoptotic dead cells and apoptotic live cells; vehicle: 17.7% ± 2.5 vs. Erucin: 28.7% ± 4.2). Cell Proliferation Assay[2] Cell Line: A549 Concentration: 0-200 µM Incubation Time: 24 h Result: Showed antiproliferative effect with an IC50 of 97.7 µM. Western Blot Analysis[2] Cell Line: A549 Concentration: 0-50 µM Incubation Time: 24 h Result: Induced the cleavage of PARP-1 at 50 µM. Increased p53 and p21 protein expression. Western Blot Analysis[3] Cell Line: RAW 264.7 Concentration: 0, 2.5, and 5 µM Incubation Time: 30 min Result: Decreased the expression of iNOS and COX-2 induced by LPS. Suppressed the LPS-induced reduction in IκB-α. Suppressed NFκB DNA binding and transcriptional activity. RT-PCR[3] Cell Line: RAW 264.7 Concentration: 0, 2.5, and 5 µM Incubation Time: 24 h Result: Decreased LPS-induced TNF-α, IL-6 and IL-1β production. |
| In Vivo | Erucin (ERU) (0-300 nM) significantly inhibits TPA-induced edema formation[3].Erucin (30 μmol/kg; i.p.; twice a week for 4 week) shows neuroprotective effects[4]. Animal Model: Female ICR mice (4 weeks of age), TPA (12-O-tetradecanoylphorbol-13-acetate)-induced mouse ear edema model[3] Dosage: 0, 100, and 300 nM Administration: Topically applied to the mouse ear 30 min prior to the topical application of TPA Result: Significantly inhibited TPA-induced edema formation. Animal Model: Male C57Bl/6 mice (9 weeks old, 25–30 g body weight)[4] Dosage: 30 μmol/kg Administration: Intraperitoneal administration, twice a week, 4 weeks (Induce brain lesion by intrastriatal injection of 6-OHDA). Result: Induced a partial recovery in the rotational behavior test. Upregulated the expression of TH. Counteract neuronal death and DNA fragmentation in 6-OHDA lesioned mice. increase total GSH and Nrf2 levels in 6-OHDA lesioned mice. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 250.6±23.0 °C at 760 mmHg |
| Melting Point | 52 °C |
| Molecular Formula | C6H11NS2 |
| Molecular Weight | 161.29 |
| Flash Point | 105.4±22.6 °C |
| Exact Mass | 161.033295 |
| PSA | 69.75000 |
| LogP | 2.39 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.531 |
| Hazard Codes | Xn |
|---|---|
| HS Code | 2930909090 |
| HS Code | 2930909090 |
|---|---|
| Summary | 2930909090. other organo-sulphur compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |