H-Lys(Tfa)-OH

H-Lys(Tfa)-OH structure
|
Common Name | H-Lys(Tfa)-OH | ||
|---|---|---|---|---|
| CAS Number | 10009-20-8 | Molecular Weight | 242.196 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 382.5±42.0 °C at 760 mmHg | |
| Molecular Formula | C8H13F3N2O3 | Melting Point | 258ºC | |
| MSDS | Chinese USA | Flash Point | 185.1±27.9 °C | |
Use of H-Lys(Tfa)-OHH-Lys(Tfa)-OH is a lysine derivative[1]. |
| Name | N6-trifluoroacetyl-L-lysine |
|---|---|
| Synonym | More Synonyms |
| Description | H-Lys(Tfa)-OH is a lysine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 382.5±42.0 °C at 760 mmHg |
| Melting Point | 258ºC |
| Molecular Formula | C8H13F3N2O3 |
| Molecular Weight | 242.196 |
| Flash Point | 185.1±27.9 °C |
| Exact Mass | 242.087830 |
| PSA | 92.42000 |
| LogP | -0.22 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.444 |
| Storage condition | 2-8°C |
| Water Solubility | 2 M HCl: 10 mg/mL, clear, colorless |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 1 |
| HS Code | 2924199090 |
|
~25% 
H-Lys(Tfa)-OH CAS#:10009-20-8 |
| Literature: Russian Journal of Organic Chemistry, , vol. 43, # 10 p. 1427 - 1431 |
|
~% 
H-Lys(Tfa)-OH CAS#:10009-20-8 |
| Literature: Journal of the Chemical Society. Perkin transactions 1, , vol. 24, p. 2349 - 2359 |
|
~63% 
H-Lys(Tfa)-OH CAS#:10009-20-8 |
| Literature: RETROTOPE, INC. Patent: WO2009/114809 A1, 2009 ; Location in patent: Page/Page column 7 ; |
| Precursor 4 | |
|---|---|
| DownStream 5 | |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Non-aqueous capillary electrophoresis 2005-2008.
Electrophoresis 30 , 36-49, (2009) This review article presents recent developments and applications of non-aqueous capillary electrophoresis (NACE): The text covers the period from the previous review (L. Geiser, J. L. Veuthey, Electr... |
|
|
Nonaqueous capillary zone electrophoresis of synthetic organic polypeptides.
Anal. Chem. 75 , 5554-5560, (2003) Poly(Nepsilon-trifluoroacetyl-L-lysine) was used as a model solute to investigate the potential of nonaqueous capillary electrophoresis (NACE) for the characterization of synthetic organic polymers. T... |
|
|
Nonaqueous capillary electrophoresis-mass spectrometry.
J. Chromatogr. A. 1159 , 28-41, (2007) Nonaqueous background electrolytes broaden the application of capillary electrophoresis displaying altered separation selectivity and interactions between analytes and buffer additives compared to aqu... |
| L-Lysine, N6-(2,2,2-trifluoroacetyl)- |
| H-Lys(Tfa)-OH |
| (S)-2-Amino-6-pivalamidohexanoic acid |
| N6-(Trifluoroacetyl)-L-lysine |
| N-(Trifluoroacetyl)-L-lysine |
| Nepsilon-Trifluoroacetyl-L-lysine |
| Nepsilon-(Trifluoroacetyl)-L-lysine |
| N(6)-trifluoroacetyl-L-lysine |
| MFCD00037223 |
| L-Lysine, N-(2,2,2-trifluoroacetyl)- |
| (2S)-2-amino-6-[(2,2,2-trifluoroacetyl)amino]hexanoic acid |
| QVYZ4MVXFFF &&L or S Form |
| Ne-Trifluoroacetyl-L-lysine |
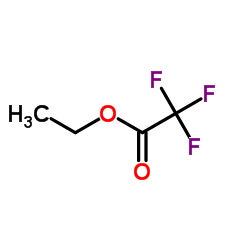
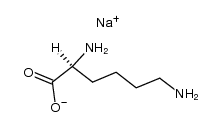
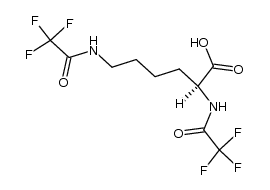
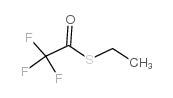

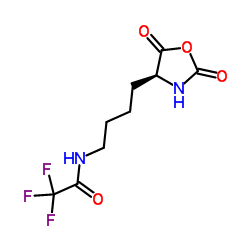 CAS#:42267-27-6
CAS#:42267-27-6 CAS#:112139-30-7
CAS#:112139-30-7![N2-[(Benzyloxy)Carbonyl]-N6-(Trifluoroacetyl)-L-Lysine structure](https://image.chemsrc.com/caspic/165/14905-30-7.png) CAS#:14905-30-7
CAS#:14905-30-7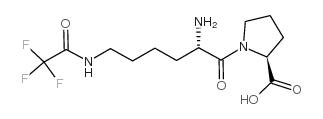 CAS#:103300-89-6
CAS#:103300-89-6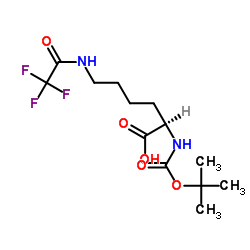 CAS#:96561-04-5
CAS#:96561-04-5
