pyridostigmine bromide
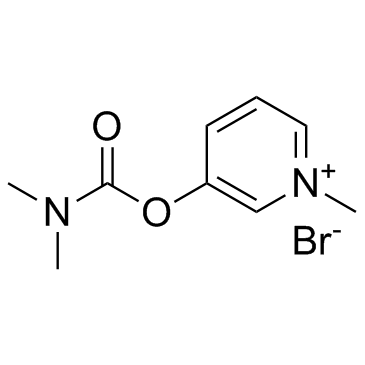
pyridostigmine bromide structure
|
Common Name | pyridostigmine bromide | ||
|---|---|---|---|---|
| CAS Number | 101-26-8 | Molecular Weight | 261.116 | |
| Density | 0.9613 g/cm3 (20ºC) | Boiling Point | 88 (25 torr) | |
| Molecular Formula | C9H13BrN2O2 | Melting Point | 154 °C | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of pyridostigmine bromidePyridostigmine is a parasympathomimetic and a reversible cholinesterase inhibitor.Target: AChEPyridostigmine is a parasympathomimetic and a reversible cholinesterase inhibitor. Since it is a quaternary amine, it is poorly absorbed in the gut and does not cross the blood–brain barrier, except possibly in stressful conditions. Pyridostigmine inhibits acetylcholinesterase in the synaptic cleft, thus slowing down the hydrolysis of acetylcholine. It is a quaternary carbamate inhibitor of cholinesterase that does not cross the blood–brain barrier which carbamylates about 30% of peripheral cholinesterase enzyme. The carbamylated enzyme eventually regenerates by natural hydrolysis and excess ACh levels revert to normal.Pyridostigmine is used to treat muscle weakness in people with myasthenia gravis and to combat the effects of curariform drug toxicity. Pyridostigmine bromide has been FDA approved for military use during combat situations as an agent to be given prior to exposure to the nerve agent Soman in order to increase survival. Used in particular during the first Gulf War, pyridostigmine bromide has been implicated as a causal factor in Gulf War syndrome. Pyridostigmine sometimes is used to treat orthostatic hypotension. It may also be of benefit in chronic axonal polyneuropathy. |
| Name | Mestinon |
|---|---|
| Synonym | More Synonyms |
| Description | Pyridostigmine is a parasympathomimetic and a reversible cholinesterase inhibitor.Target: AChEPyridostigmine is a parasympathomimetic and a reversible cholinesterase inhibitor. Since it is a quaternary amine, it is poorly absorbed in the gut and does not cross the blood–brain barrier, except possibly in stressful conditions. Pyridostigmine inhibits acetylcholinesterase in the synaptic cleft, thus slowing down the hydrolysis of acetylcholine. It is a quaternary carbamate inhibitor of cholinesterase that does not cross the blood–brain barrier which carbamylates about 30% of peripheral cholinesterase enzyme. The carbamylated enzyme eventually regenerates by natural hydrolysis and excess ACh levels revert to normal.Pyridostigmine is used to treat muscle weakness in people with myasthenia gravis and to combat the effects of curariform drug toxicity. Pyridostigmine bromide has been FDA approved for military use during combat situations as an agent to be given prior to exposure to the nerve agent Soman in order to increase survival. Used in particular during the first Gulf War, pyridostigmine bromide has been implicated as a causal factor in Gulf War syndrome. Pyridostigmine sometimes is used to treat orthostatic hypotension. It may also be of benefit in chronic axonal polyneuropathy. |
|---|---|
| Related Catalog | |
| References |
| Density | 0.9613 g/cm3 (20ºC) |
|---|---|
| Boiling Point | 88 (25 torr) |
| Melting Point | 154 °C |
| Molecular Formula | C9H13BrN2O2 |
| Molecular Weight | 261.116 |
| Exact Mass | 260.016022 |
| PSA | 33.42000 |
| Index of Refraction | 1.48 (20ºC) |
| Storage condition | Refrigerator |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300 + H310 + H330-H317 |
| Precautionary Statements | Missing Phrase - N15.00950417-P260-P262-P280-P302 + P352 + P310-P304 + P340 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T+: Very toxic; |
| Risk Phrases | R26/27/28 |
| Safety Phrases | S22-S36/37/39-S45 |
| RIDADR | UN 2811 6.1/PG 2 |
| RTECS | UU5270000 |
|
~% 
pyridostigmine ... CAS#:101-26-8 |
| Literature: Zeitschrift fuer Naturforschung, Teil B: Anorganische Chemie, Organische Chemie, , vol. 40, # 10 p. 1401 - 1408 |
|
~89% 
pyridostigmine ... CAS#:101-26-8 |
| Literature: Zeitschrift fuer Naturforschung, Teil B: Anorganische Chemie, Organische Chemie, , vol. 40, # 10 p. 1401 - 1408 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
|
Gulf War agent exposure causes impairment of long-term memory formation and neuropathological changes in a mouse model of Gulf War Illness.
PLoS ONE 10(3) , e0119579, (2015) Gulf War Illness (GWI) is a chronic multisymptom illness with a central nervous system component such as memory deficits, neurological, and musculoskeletal problems. There are ample data that demonstr... |
|
|
Gingival pain: an unusual side effect of ziprasidone.
BMJ Case Rep. 2013 , doi:10.1136/bcr-2012-007577, (2013) The patient is a 52-year-old man with schizophrenia who developed severe, unremitting gingival pain after his ziprasidone dosage was increased from 80 to 120 mg. His physical examination and laborator... |
|
|
4R-cembranoid protects against diisopropylfluorophosphate-mediated neurodegeneration.
Neurotoxicology 44 , 80-90, (2014) Many organophosphorous esters synthesized for applications in industry, agriculture, or warfare irreversibly inhibit acetylcholinesterase, and acute poisoning with these compounds causes life-threaten... |
| 3-(Dimethylcarbamoyloxy)-1-methylpyridinium Bromide |
| Pyridostigminbromid |
| mestinonbromide |
| EINECS 202-929-9 |
| ro1-5130 |
| 3-Dimethylcarbamoyloxy-1-methyl-pyridinium,Bromid |
| pyridostigmine |
| 3-dimethylcarbamoyloxy-1-methyl-pyridinium,bromide |
| MFCD00079283 |
| pyridostigmine bromide |
| Pyridinium, 3-(((dimethylamino)carbonyl)oxy)-1-methyl-, bromide |
| regonal |
| kalymin |
| Pyridinium, 3-[[(dimethylamino)carbonyl]oxy]-1-methyl-, bromide (1:1) |
| 3-Hydroxy-1 |
| 3-((Dimethylcarbamoyl)oxy)-1-methylpyridin-1-ium bromide |
| mestinonebromide |
| pyridostygmine bromide |
| 3-[(Dimethylcarbamoyl)oxy]-1-methylpyridinium bromide |
| 3-[[(Dimethylamino)carbonyl]oxy]-1-methylpyridinium Bromide |
| Pyridostigmine (bromide) |
| 3-{[(dimethylamino)carbonyl]oxy}-1-methylpyridinium bromide |
| pyridinium, 3-[[(dimethylamino)carbonyl]oxy]-1-methyl-, bromide |
| kalimin |
| Mestinon |
| PYBR |