Cinnamyl alcohol
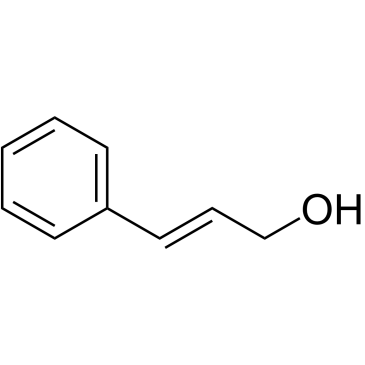
Cinnamyl alcohol structure
|
Common Name | Cinnamyl alcohol | ||
|---|---|---|---|---|
| CAS Number | 104-54-1 | Molecular Weight | 134.175 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 250.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C9H10O | Melting Point | 30-33 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 124.8±14.5 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Cinnamyl alcoholCinnamyl Alcohol is an active component from chestnut flower, inhibits increased PPARγ expression, with anti-obesity activity[1]. |
| Name | cinnamyl alcohol |
|---|---|
| Synonym | More Synonyms |
| Description | Cinnamyl Alcohol is an active component from chestnut flower, inhibits increased PPARγ expression, with anti-obesity activity[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 250.0±0.0 °C at 760 mmHg |
| Melting Point | 30-33 °C(lit.) |
| Molecular Formula | C9H10O |
| Molecular Weight | 134.175 |
| Flash Point | 124.8±14.5 °C |
| Exact Mass | 134.073166 |
| PSA | 20.23000 |
| LogP | 1.70 |
| Vapour density | 4.6 (vs air) |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.599 |
| Storage condition | 2-8°C |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| Water Solubility | 1.8 g/L (20 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H317-H319 |
| Precautionary Statements | P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22;R36;R43 |
| Safety Phrases | S26-S36/37-S37/39-S24 |
| RIDADR | 2811 |
| WGK Germany | 2 |
| RTECS | GE2200000 |
| Hazard Class | 6.1 |
| HS Code | 2912299000 |
| Precursor 9 | |
|---|---|
| DownStream 9 | |
| HS Code | 2912299000 |
|---|---|
| Summary | 2912299000. other cyclic aldehydes without other oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:5.5%. General tariff:30.0% |
|
Parallel dual secondary column-dual detection: a further way of enhancing the informative potential of two-dimensional comprehensive gas chromatography.
J. Chromatogr. A. 1360 , 264-74, (2014) Comprehensive two-dimensional gas chromatography (GC×GC) coupled with Mass Spectrometry (MS) is one of today's most powerful analytical platforms for detailed analysis of medium-to-high complexity sam... |
|
|
Functional characterization of two acyltransferases from Populus trichocarpa capable of synthesizing benzyl benzoate and salicyl benzoate, potential intermediates in salicinoid phenolic glycoside biosynthesis.
Phytochemistry 113 , 149-59, (2015) Salicinoids are phenolic glycosides (PGs) characteristic of the Salicaceae and are known defenses against insect herbivory. Common examples are salicin, salicortin, tremuloidin, and tremulacin, which ... |
|
|
Contribution of cinnamic acid analogues in rosmarinic acid to inhibition of snake venom induced hemorrhage.
Bioorg. Med. Chem. 19 , 2392-6, (2011) In our previous paper, we reported that rosmarinic acid (1) of Argusia argentea could neutralize snake venom induced hemorrhagic action. Rosmarinic acid (1) consists of two phenylpropanoids: caffeic a... |
| cinnamic alcohol |
| stylone |
| trans-3-phenyl-2-propen-1-ol |
| Peruvin |
| Ciamyl alcohol |
| STYRYL CARBINOL |
| Cinnamylalkohol |
| (E)-cinnamyl alcohol |
| (E)-3-phenylprop-2-en-1-ol |
| 3-PHENYLALLYLOL |
| EINECS 203-212-3 |
| (2E)-3-phenylprop-2-en-1-ol |
| Sterone |
| MFCD00002921 |
| (2E)-3-Phenyl-2-propen-1-ol |
| Cinnamyl alcohol |
| 3-phenylprop-2-en-1-ol |
| 3-Phenyl |
| allylic benzylic alcohol |
| cinnamyl alcohol, (E)-isomer |
| Chnnamyl alcohol |
| cynnamyl alcohol |
| Styrylicalcohol |
| 3-phenyl-2-propen-1-ol |
| 2-Propen-1-ol, 3-phenyl-, (2E)- |
 CAS#:1504-58-1
CAS#:1504-58-1 CAS#:4393-06-0
CAS#:4393-06-0![Benzene, [(1E)-3-(methoxymethoxy)-1-propen-1-yl] Structure](https://image.chemsrc.com/caspic/483/88738-40-3.png) CAS#:88738-40-3
CAS#:88738-40-3 CAS#:104-55-2
CAS#:104-55-2 CAS#:621-82-9
CAS#:621-82-9 CAS#:103-36-6
CAS#:103-36-6 CAS#:109283-53-6
CAS#:109283-53-6 CAS#:71700-50-0
CAS#:71700-50-0 CAS#:102-92-1
CAS#:102-92-1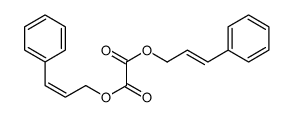 CAS#:111944-30-0
CAS#:111944-30-0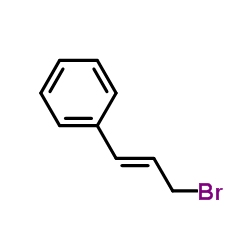 CAS#:4392-24-9
CAS#:4392-24-9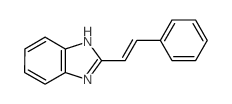 CAS#:1456-19-5
CAS#:1456-19-5 CAS#:2243-52-9
CAS#:2243-52-9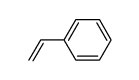 CAS#:292638-84-7
CAS#:292638-84-7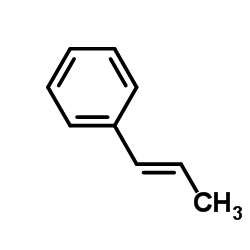 CAS#:637-50-3
CAS#:637-50-3 CAS#:63576-88-5
CAS#:63576-88-5
