Manzamine A hydrochloride
Modify Date: 2024-01-11 10:34:45
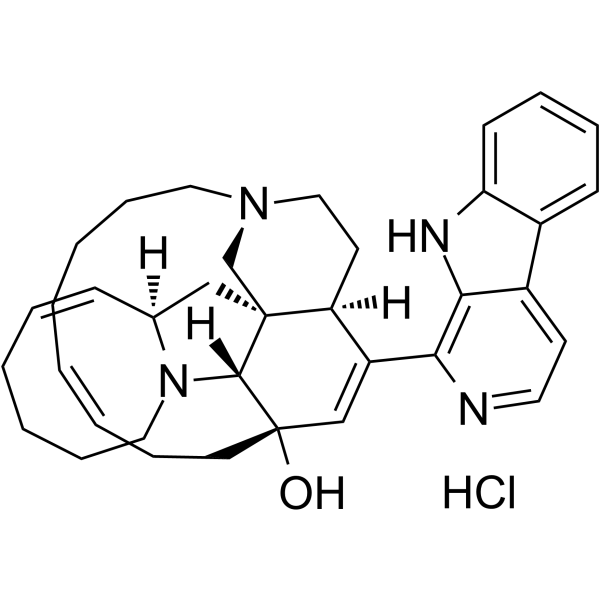
Manzamine A hydrochloride structure
|
Common Name | Manzamine A hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 104264-80-4 | Molecular Weight | 585.22 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C36H45ClN4O | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Manzamine A hydrochlorideManzamine A hydrochloride, an orally active beta-carboline alkaloid, inhibits specifically GSK-3β and CDK-5 with IC50s of 10.2 and 1.5μM, respectively. Manzamine A hydrochloride targets vacuolar ATPases and inhibits autophagy in pancreatic cancer cells. Manzamine A hydrochloride has antimalarial and anticancer activities. Manzamine A hydrochloride also shows potent activity against HSV-1[1][2][3][4]. |
| Name | Manzamine A hydrochloride |
|---|
| Description | Manzamine A hydrochloride, an orally active beta-carboline alkaloid, inhibits specifically GSK-3β and CDK-5 with IC50s of 10.2 and 1.5μM, respectively. Manzamine A hydrochloride targets vacuolar ATPases and inhibits autophagy in pancreatic cancer cells. Manzamine A hydrochloride has antimalarial and anticancer activities. Manzamine A hydrochloride also shows potent activity against HSV-1[1][2][3][4]. |
|---|---|
| Related Catalog | |
| Target |
GSK-3β:10.2 μM (IC50) CDK5:1.5 μM (IC50) HIV-1 vacuolar ATPases Malaria |
| In Vitro | Manzamine A increases acidity in pancreatic cancer cells and non-malignant Vero cells. manzamine A is a potential inhibitor of autophagy by preventing autophagosome turnover. Manzamine A (10 µM; 2 hours; AsPC-1 cells) clearly induced an accumulation of p62 confirming an inhibition of autophagosome turnover[2]. Manzamine A represents an important lead structure for the development of novel antimalarial chemotherapies[3]. |
| References |
| Molecular Formula | C36H45ClN4O |
|---|---|
| Molecular Weight | 585.22 |