Adefovir
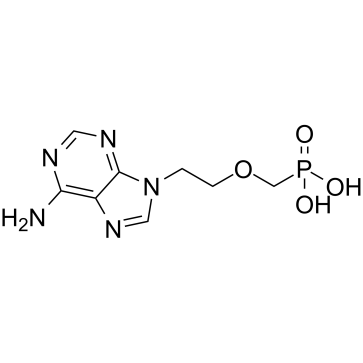
Adefovir structure
|
Common Name | Adefovir | ||
|---|---|---|---|---|
| CAS Number | 106941-25-7 | Molecular Weight | 273.186 | |
| Density | 1.9±0.1 g/cm3 | Boiling Point | 632.5±65.0 °C at 760 mmHg | |
| Molecular Formula | C8H12N5O4P | Melting Point | >260°C | |
| MSDS | Chinese USA | Flash Point | 336.3±34.3 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of AdefovirAdefovir (GS-0393) is an adenosine monophosphate analog antiviral agent that after intracellular conversion to Adefovir diphosphate inhibits HBV DNA polymerase. Adefovir has an IC50 of 0.7 μM against HBV in the HepG2.2.15 cell line. Adefovir has good antiviral activity against several viruses, including HBV and herpesviruses[1][2][3]. |
| Name | Adefovir |
|---|---|
| Synonym | More Synonyms |
| Description | Adefovir (GS-0393) is an adenosine monophosphate analog antiviral agent that after intracellular conversion to Adefovir diphosphate inhibits HBV DNA polymerase. Adefovir has an IC50 of 0.7 μM against HBV in the HepG2.2.15 cell line. Adefovir has good antiviral activity against several viruses, including HBV and herpesviruses[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
HBV[1][2][3]; DNA polymerase[1][2] |
| In Vitro | Studies to elucidate the mechanism of action of Adefovir against herpesvirus replication reveals that the phosphorylation of Adefovir occurred intracellularly and is carried out by host cellular enzymes. The diphosphorylated derivatives of Adefovir targeted the viral DNA polymerase and also acted as DNA chain terminators. Adenylate kinase is shown to be responsible for the first phosphorylation, which was followed by ADP kinase and creatine kinase, forming Adefovir diphosphate[1]. |
| In Vivo | Unaffected by food, Adefovir achieves 60% oral bioavailability. Its half-life is 12-30 hours and Adefovir undergoes renal excretion without significant metabolites. Adefovir does not substantially affect the cytochrome P450 system[3]. |
| References |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 632.5±65.0 °C at 760 mmHg |
| Melting Point | >260°C |
| Molecular Formula | C8H12N5O4P |
| Molecular Weight | 273.186 |
| Flash Point | 336.3±34.3 °C |
| Exact Mass | 273.062683 |
| PSA | 146.19000 |
| LogP | -2.06 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.769 |
| Storage condition | -20°C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S26 |
| RIDADR | UN 2585 8/PG 3 |
| WGK Germany | 2 |
| RTECS | SZ6563500 |
| Packaging Group | III |
| Hazard Class | 8 |
| HS Code | 2933990090 |
| Precursor 9 | |
|---|---|
| DownStream 6 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
In vitro evaluation of 9-(2-phosphonylmethoxyethyl)adenine ester analogues, a series of anti-HBV structures with improved plasma stability and liver release.
Arch. Pharm. Res. 37(11) , 1416-25, (2014) Chronic hepatitis B virus (HBV) infection may lead to liver cirrhosis and hepatocellular carcinoma, but few drugs are available for its treatment. Acyclic nucleoside phosphonates (ANPs) have remarkabl... |
|
|
Quantitative high-throughput identification of drugs as modulators of human constitutive androstane receptor.
Sci. Rep. 5 , 10405, (2015) The constitutive androstane receptor (CAR, NR1I3) plays a key role in governing the transcription of numerous hepatic genes that involve xenobiotic metabolism/clearance, energy homeostasis, and cell p... |
|
|
Surface engineered polymeric nanocarriers mediate the delivery of transferrin-methotrexate conjugates for an improved understanding of brain cancer.
Biochem. Biophys. Res. Commun. 457(3) , 353-7, (2015) The objective of present study was to enhance permeation of bioactive molecules across blood brain barrier (BBB) through polysorbate 80 coated poly-lactic-co-glycolic acid (PLGA) nanoparticles (NPs) l... |
| gs0393 |
| ((2-(6-Amino-9H-purin-9-yl)ethoxy)methyl)phosphonic acid |
| MFCD00866943 |
| PMEA |
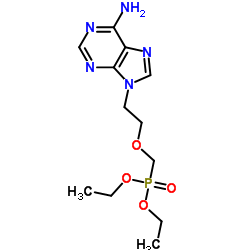 CAS#:116384-53-3
CAS#:116384-53-3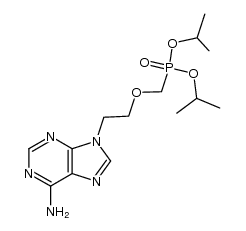 CAS#:142340-94-1
CAS#:142340-94-1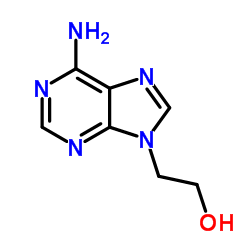 CAS#:707-99-3
CAS#:707-99-3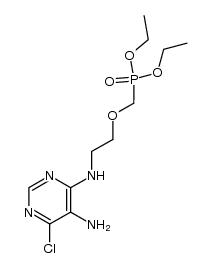 CAS#:212894-84-3
CAS#:212894-84-3 CAS#:185900-69-0
CAS#:185900-69-0 CAS#:66224-66-6
CAS#:66224-66-6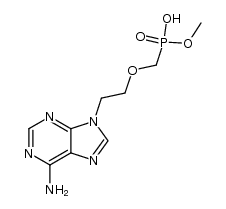 CAS#:107021-27-2
CAS#:107021-27-2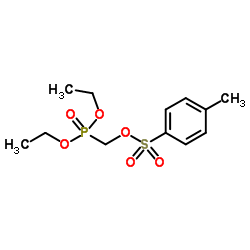 CAS#:31618-90-3
CAS#:31618-90-3![{[2-(6-amino-9H-purin-9-yl)ethoxy]methyl}phosphinic acid Structure](https://image.chemsrc.com/caspic/107/159531-21-2.png) CAS#:159531-21-2
CAS#:159531-21-2![9-[2-(dichlorophosphorylmethoxy)ethyl]purin-6-amine structure](https://image.chemsrc.com/caspic/498/142341-38-6.png) CAS#:142341-38-6
CAS#:142341-38-6 CAS#:142340-99-6
CAS#:142340-99-6![9-[2-({bis[(pivaloyloxy)methoxy]phosphinyl}methoxy)ethyl]-6-[(hydroxymethyl)amino]purine structure](https://image.chemsrc.com/caspic/436/323201-04-3.png) CAS#:323201-04-3
CAS#:323201-04-3![9-[2-(diphenoxyphosphorylmethoxy)ethyl]purin-6-amine structure](https://image.chemsrc.com/caspic/357/142341-24-0.png) CAS#:142341-24-0
CAS#:142341-24-0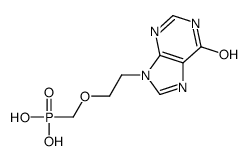 CAS#:113884-65-4
CAS#:113884-65-4
