HALOFENOZIDE
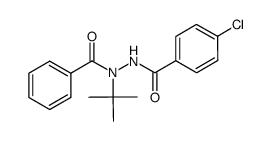
HALOFENOZIDE structure
|
Common Name | HALOFENOZIDE | ||
|---|---|---|---|---|
| CAS Number | 112226-61-6 | Molecular Weight | 330.81 | |
| Density | 1.205g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C18H19ClN2O2 | Melting Point | 198.0-199.0° | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |


GHS07, GHS09 |
Signal Word | Warning | |
Use of HALOFENOZIDEHalofenozide (RH-0345) is an ecdysteroid agonist. RH-0345 belongs to a new group of insect growth regulators (IGRs) with a benzoylhydrazine structure that mimic the action of the natural insect molting hormone 20-hydroxyecdysone[1]. |
| Name | halofenozide |
|---|---|
| Synonym | More Synonyms |
| Description | Halofenozide (RH-0345) is an ecdysteroid agonist. RH-0345 belongs to a new group of insect growth regulators (IGRs) with a benzoylhydrazine structure that mimic the action of the natural insect molting hormone 20-hydroxyecdysone[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Halofenozide (RH-0345) reduces both growth and development of oocytes in vitro[1]. Cell Viability Assay[2] Cell Line: Cultured ovaries were dissected from female adult beetles of mealworm, Tenebrio molitor (T. molitor) Concentration: 1 μM and 10 μM Incubation Time: 4 days Result: Did not cause a significant effect on ecdysteroid amounts in the culture medium using ovaries from 0- and 2-day-old females at 10 μM. Caused a small significant increase of the amounts of ovarian ecdysteroids in the culture medium at 1 μM in the culture medium and with 2-day-old ovaries. |
| In Vivo | After topical application of Halofenozide (RH-0345; 5 and 10 μg) on adult females, the highest dose (10 μg) reduces significantly the reproductive parameters scored: namely the oviposition period, the fecundity and the egg viability[1]. Halofenozide at the two doses tested (5 and 10 μg) causes smaller eggs with a decrease in length, width and volume[1]. Animal Model: Newly emerged (0-day-old) adult females of T. molitor [1] Dosage: 5 and 10 μg Administration: Topically applied Result: Caused smaller eggs with a decrease in length, width and volume at the two doses tested (5 and 10 μg) . |
| References |
| Density | 1.205g/cm3 |
|---|---|
| Melting Point | 198.0-199.0° |
| Molecular Formula | C18H19ClN2O2 |
| Molecular Weight | 330.81 |
| Exact Mass | 330.11400 |
| PSA | 49.41000 |
| LogP | 4.31660 |
| Index of Refraction | 1.584 |
|
~% 
HALOFENOZIDE CAS#:112226-61-6 |
| Literature: EP982292 A2, ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Two Leptinotarsa uridine diphosphate N-acetylglucosamine pyrophosphorylases are specialized for chitin synthesis in larval epidermal cuticle and midgut peritrophic matrix.
Insect Biochem. Mol. Biol. 68 , 12-Jan, (2016) Uridine diphosphate-N-acetylglucosamine-pyrophosphorylase (UAP) is involved in the biosynthesis of chitin, an essential component of the epidermal cuticle and midgut peritrophic matrix (PM) in insects... |
| RH 0345 |
| N-tert-butyl-N’-(4-chlorobenzoyl)benzohydrazide |
| N-tert-Butyl-N'-(4-chlorobenzoyl)benzohydrazide |
| N'-benzoyl-N'-tert-butyl-4-chlorobenzohydrazide |
| 4-chlorobenzoic acid 2-benzoyl-2-(1,1-dimethylethyl)hydrazide |
| Mach 2 |
| Halofenozide |
| N’-benzoyl-N’-tert-butyl-4-chlorobenzohydrazide |