Isolongifolene
Modify Date: 2024-01-02 11:07:13

Isolongifolene structure
|
Common Name | Isolongifolene | ||
|---|---|---|---|---|
| CAS Number | 1135-66-6 | Molecular Weight | 204.351 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 266.5±7.0 °C at 760 mmHg | |
| Molecular Formula | C15H24 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 102.6±13.0 °C | |
Use of IsolongifoleneIsolongifolene ((-)-Isolongifolene) is a tricyclic sesquiterpene isolated from Murraya koenigii. Isolongifolene attenuates Rotenone-induced oxidative stress, mitochondrial dysfunction and apoptosis through the regulation of P13K/AKT/GSK-3β signaling pathways. Isolongifolene has antioxidant, anti-inflammatory, anticancer and neuroprotective properties[1]. |
| Name | Isolongifolene |
|---|---|
| Synonym | More Synonyms |
| Description | Isolongifolene ((-)-Isolongifolene) is a tricyclic sesquiterpene isolated from Murraya koenigii. Isolongifolene attenuates Rotenone-induced oxidative stress, mitochondrial dysfunction and apoptosis through the regulation of P13K/AKT/GSK-3β signaling pathways. Isolongifolene has antioxidant, anti-inflammatory, anticancer and neuroprotective properties[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Isolongifolene (0-50 μM; 26 hours; SH-SY5Y neuroblastoma cells) treatment significantly alleviates Rotenone-induced cytotoxicity in SH-SY5Y cells in a dose-dependent manner[1]. Isolongifolene (10 μM; 26 hours; SH-SY5Y neuroblastoma cells) treatment attenuates Rotenone-induced apoptosis in SH-SY5Y cells[1]. Isolongifolene (10 μM; 26 hours; SH-SY5Y neuroblastoma cells) treatment attenuates Rotenone induced toxicity by down-regulating Bax, caspases-3, 6, 8 and 9 expression and up-regulating of Bcl-2 expression. Furthermore regulation of p-P13K, p-AKT and p-GSK-3β expression by Isolongifolene[1]. Cell Viability Assay[1] Cell Line: SH-SY5Y neuroblastoma cells Concentration: 0 μM, 1 μM, 2.5 μM, 5 μM, 10 μM, 20 μM and 50 µM Incubation Time: 26 hours Result: Significantly alleviated Rotenone-induced cytotoxicity in SH-SY5Y cells. Apoptosis Analysis[1] Cell Line: SH-SY5Y neuroblastoma cells Concentration: 10 µM Incubation Time: 26 hours Result: Attenuated Rotenone-induced apoptosis in SH-SY5Y cells. Western Blot Analysis[1] Cell Line: SH-SY5Y neuroblastoma cells Concentration: 10 µM Incubation Time: 26 hours Result: Attenuated rotenone induced toxicity by down-regulating Bax, caspases-3, 6, 8 and 9 expression and up-regulating of Bcl-2 expression. Prevented the rotenone-induced decreased phosphorylation of GSK-3β. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 266.5±7.0 °C at 760 mmHg |
| Molecular Formula | C15H24 |
| Molecular Weight | 204.351 |
| Flash Point | 102.6±13.0 °C |
| Exact Mass | 204.187805 |
| LogP | 6.36 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.518 |
| Precursor 8 | |
|---|---|
| DownStream 1 | |
| HS Code | 2902199090 |
|---|---|
| Summary | 2902199090 other cyclanes, cyclenes and cyclotherpenes。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:2.0%。General tariff:30.0% |
| 2H-2,4a-Methanonaphthalene, 1,3,4,5,6,7-hexahydro-1,1,5,5-tetramethyl- |
| 2H-2,4a-Methanonaphthalene, 1,3,4,5,6,7-hexahydro-1,1,5,5-tetramethyl-, (2S,4aR)- |
| (1R)-2,2,7,7-Tetramethyltricyclo[6.2.1.01.6]undec-5-ene |
| EINECS 214-494-2 |
| (1R,8S)-2,2,7,7-Tetramethyltricyclo[6.2.1.0]undec-5-ene |
| 2H-2,4a-Methanonaphthalene, 1,3,4,5,6,7-hexahydro-1,1,5,5-tetramethyl-, (2S,4aR)-(-)- |
| 2,2,7,7-Tetramethyltricyclo[6.2.1.0]undec-5-ene |
| trans-Isolongifolene |
| 1,3,4,5,6,7-hexahydro-1,1,5,5-tetramethyI-2H-2,4a-methanonaphthalene |
| MFCD00042616 |
| UNII:PX6N25M90H |
| iso-Longifolene |
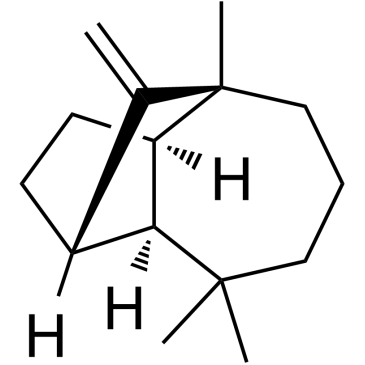 CAS#:475-20-7
CAS#:475-20-7 CAS#:16846-09-6
CAS#:16846-09-6 CAS#:70913-79-0
CAS#:70913-79-0 CAS#:5989-08-2
CAS#:5989-08-2 CAS#:1139-17-9
CAS#:1139-17-9 CAS#:108643-05-6
CAS#:108643-05-6 CAS#:108643-01-2
CAS#:108643-01-2 CAS#:108643-04-5
CAS#:108643-04-5![1-(octahydro-7,7,8,8-tetramethyl-2,3b-methano-3bH-cyclopenta[1,3]cyclopropa[1,2]benzen-4-yl)ethanone structure](https://image.chemsrc.com/caspic/433/59056-72-3.png) CAS#:59056-72-3
CAS#:59056-72-3