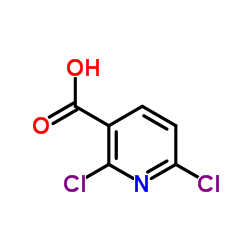
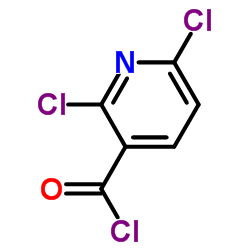
CX-5461
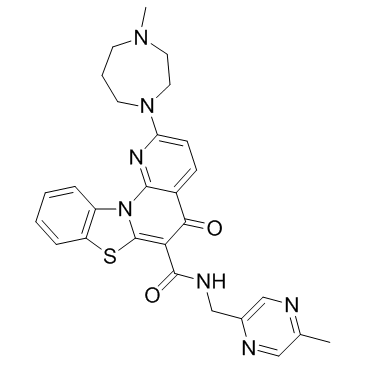
CX-5461 structure
|
Common Name | CX-5461 | ||
|---|---|---|---|---|
| CAS Number | 1138549-36-6 | Molecular Weight | 513.614 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 739.9±60.0 °C at 760 mmHg | |
| Molecular Formula | C27H27N7O2S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 401.3±32.9 °C | |
Use of CX-5461CX-5461 is a potent and oral rRNA synthesis inhibitor. It inhibits RNA polymerase I-driven transcription of rRNA with IC50s of 142, 113, and 54 nM in HCT-116, A375, and MIA PaCa-2 cells, respectively. |
| Name | 2-(4-methyl-1,4-diazepan-1-yl)-N-[(5-methylpyrazin-2-yl)methyl]-5-oxo-[1,3]benzothiazolo[3,2-a][1,8]naphthyridine-6-carboxamide |
|---|---|
| Synonym | More Synonyms |
| Description | CX-5461 is a potent and oral rRNA synthesis inhibitor. It inhibits RNA polymerase I-driven transcription of rRNA with IC50s of 142, 113, and 54 nM in HCT-116, A375, and MIA PaCa-2 cells, respectively. |
|---|---|
| Related Catalog | |
| Target |
IC50: 54 nM (rRNA synthesis, MIA PaCa-2 cells), 113 nM (rRNA synthesis, A375 cells), 142 nM (rRNA synthesis, HCT-116 cells)[1] |
| In Vitro | CX-5461 is a potent and orally bioavailable inhibitor of Pol I-mediated rRNA synthesis, with IC50s of 142 nM in HCT-116, 113 nM in A375, and 54 nM in MIA PaCa-2 cells, and shows little or no effect on Pol II (IC50, ≥25 μM). CX-5461 has modest inhibition on DNA replication and protein translation. CX-5461 also exhibits broad antiproliferative activity against a panel of human cancer cell lines, with a mean EC50 of 147 nM, but has minimal effect on viability of nontransformed human cells, with EC50 values of appr 5000 nM. EC50s of CX-5461 for HCT-116, A375, and MIA PaCa-2 cell lines are 167, 58, and 74 nM, respectively. CX-5461 induces autophagy and senescence in solid tumor cancer cells, rather than apoptosis, through a p53-independent process[1]. Eμ-Myc lymphoma cells from tumor-bearing mice are exquisitely sensitive to CX-5461 with an IC50 of 27.3 nM ± 8.1 nM for Pol I transcription after 1 hr and IC50 of 5.4 nM ± 2.1 nM for cell death after 16 hr. CX-5461 activates p53 via the nucleolar stress response in Eμ-MycLymphoma Cells[2]. |
| In Vivo | CX-5461 displays antitumor activity against human solid tumors in murine xenograft models. CX-5461 (50 mg/kg, p.o.) shows significant MIA PaCa-2 growth inhibition with TGI equal to 69% on day 31 and 79% TGI on A375 on day 32[1]. CX-5461 (50 mg/kg, p.o.) inhibits the Eμ-Myc tumor cells with 84% repression in Pol I transcription at 1 hr posttreatment in C57BL/6 mice. CX-5461 also induces a rapid reduction in tumor burden in the lymph nodes and a concomitant reduction of spleen size to within the normal range[2]. |
| Cell Assay | Cells are plated on 96-well plates and treated the next day with dose response of CX-5461 for 96 hours. Cell viability is determined using Alamar Blue and CyQUANT assays[1]. |
| Animal Admin | Mice[1] Animal experiments are performed with 5- to 6-week-old female athymic (NCr nu/nu fisol) mice of Balb/c. Mice are inoculated with athymic (NCr nu/nu fisol) mice in 100 μL of cell suspension subcutaneously in the right flank. Tumor measurements are performed by caliper analysis, and tumor volume is calculated using the formula (l×w2)/2, where w=width and l=length in mm of the tumor. established tumors (appr 110-120 mm3) are randomized into vehicle (50 mM NaH2PO4, pH 4.5), gemcitabine, or CX-5461 treatment groups. Tumor growth inhibition (TGI) is determined on the last day of study according to the formula: TGI (%)=[100 − (VfD− ViD)/ (VfV − ViV) × 100], where ViV is the initial mean tumor volume in vehicle-treated group, VfV is the final mean tumor volume in vehicle-treated group, ViD is the initial mean tumor volume in drug-treated group, and VfD is the final mean tumor volume in drug-treated group. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 739.9±60.0 °C at 760 mmHg |
| Molecular Formula | C27H27N7O2S |
| Molecular Weight | 513.614 |
| Flash Point | 401.3±32.9 °C |
| Exact Mass | 513.194702 |
| PSA | 123.97000 |
| LogP | -0.81 |
| Vapour Pressure | 0.0±2.4 mmHg at 25°C |
| Index of Refraction | 1.740 |
| Storage condition | -20°C |
|
~87% 
CX-5461 CAS#:1138549-36-6 |
| Literature: Schwaebe, Michael K.; Ryckman, David M.; Nagasawa, Johnny Y.; Pierre, Fabrice; Vialettes, Anne; Haddach, Mustapha Tetrahedron Letters, 2011 , vol. 52, # 10 p. 1096 - 1100 |
|
~% 
CX-5461 CAS#:1138549-36-6 |
| Literature: ACS Medicinal Chemistry Letters, , vol. 3, # 7 p. 602 - 606 |
|
~% 
CX-5461 CAS#:1138549-36-6 |
| Literature: ACS Medicinal Chemistry Letters, , vol. 3, # 7 p. 602 - 606 |
|
~% 
CX-5461 CAS#:1138549-36-6 |
| Literature: ACS Medicinal Chemistry Letters, , vol. 3, # 7 p. 602 - 606 |
| UNII-3R4C5YLB9I |
| CS-0568 |
| pound not CX5461 pound not CX 5461 |
| 2-(4-methyl[1,4]diazepan-1-yl)-5-oxo-5H-7-thia-1,11b-diaza-benzo[c]fluorene-6-carboxylic acid (5-methyl-pyrazin-2-ylmethyl)amide |
| 2-(4-Methyl-1,4-diazepan-1-yl)-N-[(5-methyl-2-pyrazinyl)methyl]-5-oxo-5H-[1,3]benzothiazolo[3,2-a][1,8]naphthyridine-6-carboxamide |
| cc-1 |
| 5H-Benzothiazolo[3,2-a][1,8]naphthyridine-6-carboxamide, 2-(hexahydro-4-methyl-1H-1,4-diazepin-1-yl)-N-[(5-methyl-2-pyrazinyl)methyl]-5-oxo- |
| CX-5461 |
| CX5461 |
![ethyl 2-(4-methyl-1,4-diazepan-1-yl)-5-oxo-5H-benzo[4,5]thiazolo[3,2-a][1,8]naphthyridine-6-carboxylate structure](https://image.chemsrc.com/caspic/188/1138555-04-0.png)
![Ethyl 2-chloro-5-oxo-5H-benzo[4,5]thiazolo[3,2-a][1,8]naphthyridine-6-carboxylate structure](https://image.chemsrc.com/caspic/089/881633-93-8.png)