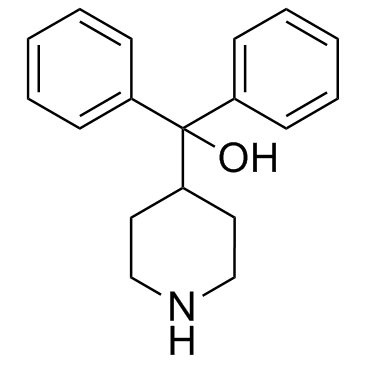
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
TN0470000
-
CHEMICAL NAME :
-
4-Piperidinemethanol, alpha,alpha-diphenyl-
-
CAS REGISTRY NUMBER :
-
115-46-8
-
BEILSTEIN REFERENCE NO. :
-
0230221
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
4
-
MOLECULAR FORMULA :
-
C18-H21-N-O
-
MOLECULAR WEIGHT :
-
267.40
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
650 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
PSCBAY Psychopharmacology Service Center, Bulletin. (Bethesda, MD) V.1-3, 1961-65. For publisher information, see PSYBB9. Volume(issue)/page/year: 2,17,1963
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
220 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
PSCBAY Psychopharmacology Service Center, Bulletin. (Bethesda, MD) V.1-3, 1961-65. For publisher information, see PSYBB9. Volume(issue)/page/year: 2,17,1963
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
350 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
PSCBAY Psychopharmacology Service Center, Bulletin. (Bethesda, MD) V.1-3, 1961-65. For publisher information, see PSYBB9. Volume(issue)/page/year: 2,17,1963
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
177 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
PSCBAY Psychopharmacology Service Center, Bulletin. (Bethesda, MD) V.1-3, 1961-65. For publisher information, see PSYBB9. Volume(issue)/page/year: 2,17,1963
|
 CAS#:114399-88-1
CAS#:114399-88-1 CAS#:96067-93-5
CAS#:96067-93-5 CAS#:108-20-3
CAS#:108-20-3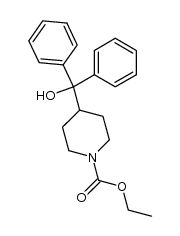 CAS#:112818-77-6
CAS#:112818-77-6 CAS#:108-86-1
CAS#:108-86-1 CAS#:24228-40-8
CAS#:24228-40-8 CAS#:1126-09-6
CAS#:1126-09-6 CAS#:160809-38-1
CAS#:160809-38-1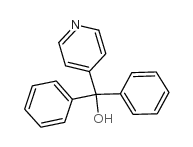 CAS#:1620-30-0
CAS#:1620-30-0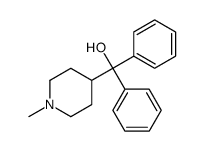 CAS#:6071-92-7
CAS#:6071-92-7![4-[4-[hydroxy(diphenyl)methyl]piperidin-1-yl]butanal structure](https://image.chemsrc.com/caspic/241/105955-84-8.png) CAS#:105955-84-8
CAS#:105955-84-8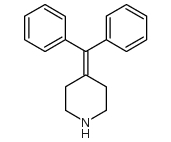 CAS#:50706-57-5
CAS#:50706-57-5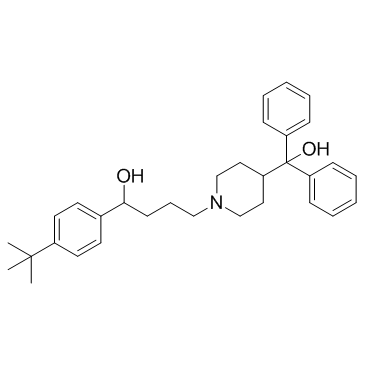 CAS#:50679-08-8
CAS#:50679-08-8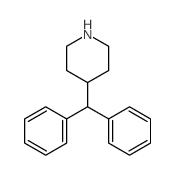 CAS#:19841-73-7
CAS#:19841-73-7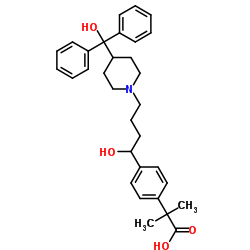 CAS#:83799-24-0
CAS#:83799-24-0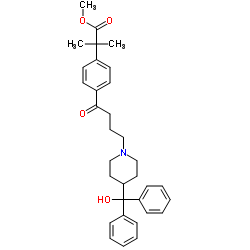 CAS#:154477-55-1
CAS#:154477-55-1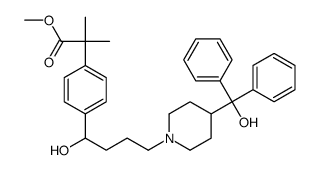 CAS#:154825-96-4
CAS#:154825-96-4![ETHYL 4-[4-[4-(HYDROXYDIPHENYLMETHYL)-1-PIPERIDINYL]-1 structure](https://image.chemsrc.com/caspic/067/169280-33-5.png) CAS#:169280-33-5
CAS#:169280-33-5![4'-tert-butyl-4-[4-(hydroxybenzhydryl)piperidino]butyrophenone structure](https://image.chemsrc.com/caspic/109/43076-30-8.png) CAS#:43076-30-8
CAS#:43076-30-8 CAS#:5704-22-3
CAS#:5704-22-3