115-46-8
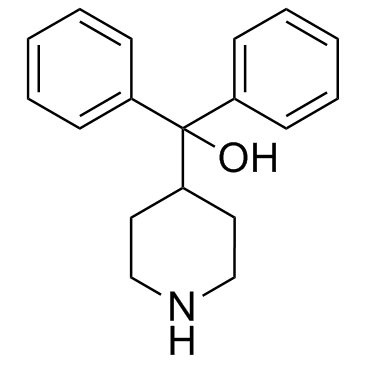
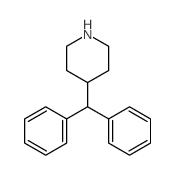
| Name | Diphenyl(piperidin-4-yl)methanol |
|---|---|
| Synonyms |
alpha,alpha-Diphenyl-4-piperidinomethanol
4-(α,α Diphenyl) piperidine methanol Diphenyl(4-piperidinyl)methanol a,a-Diphenyl-4-piperidinemethanol γ-Pipradrol MFCD00066980 Ataractan 4-Piperidinemethanol, α,α-diphenyl- α,α-Diphenyl-4-piperidinemethanol α-(4-Piperidyl)benzhydrol azacyclonol diphenyl(4-piperidyl)methanol Psychosan g-Pipradrol diphenyl(piperidin-4-yl)methanol α,α-Diphenyl-4-piperidinomethanol Diphenyl (g-Pyridyl)carbinol a-(4-Piperidyl)benzhydrol 4-(Diphenylhydroxymethyl)piperidine EINECS 204-092-5 Calmeran |
| Description | Azacyclonol, also known as γ-pipradol, is a drug used to diminish hallucinations in psychotic individuals.Target: OthersAzacyclonol is a drug which is a so-called ataractive, or agent which diminishes hallucinations in psychotic individuals. The formation of Azacyclonol in human intestinal microsomes is linear with respect to time up to 60 min. The rates of formation of Azacyclonol increases linearly with microsomal protein concentration up to 2 mg/mL. The apparent Km and Vmax values of Azacyclonol are 0.82 μM and 60 pmol/min/mg protein in microsomes from human liver [1]. The formation of Azacyclonol and terfenadine alcohol from terfenadine is confirmed to be catalyzed predominantly by CYP3A(4) isozyme, and the ratio of the rate of terfenadine alcohol formation to that of Azacyclonol is 3:1 [2]. The amount of Azacyclonol eliminated renally increases on average 2-fold after rifampin dosing [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 445.5±40.0 °C at 760 mmHg |
| Melting Point | 160-163 °C |
| Molecular Formula | C18H21NO |
| Molecular Weight | 267.365 |
| Flash Point | 142.0±18.0 °C |
| Exact Mass | 267.162323 |
| PSA | 32.26000 |
| LogP | 3.25 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.584 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi:Irritant |
|---|---|
| Risk Phrases | R36/37/38 |
| Safety Phrases | S24/25 |
| RTECS | TN0470000 |
| HS Code | 29333999 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |


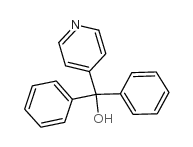
![4-[4-[hydroxy(diphenyl)methyl]piperidin-1-yl]butanal structure](https://image.chemsrc.com/caspic/241/105955-84-8.png)
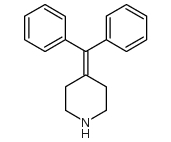
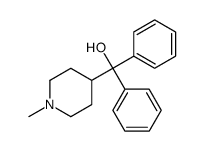
![ETHYL 4-[4-[4-(HYDROXYDIPHENYLMETHYL)-1-PIPERIDINYL]-1 structure](https://image.chemsrc.com/caspic/067/169280-33-5.png)
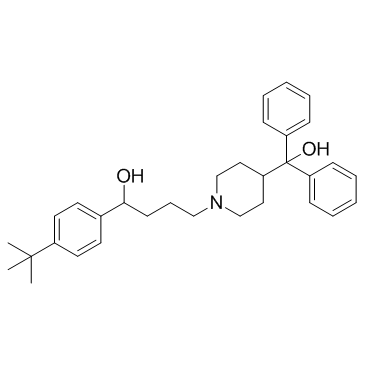
![4'-tert-butyl-4-[4-(hydroxybenzhydryl)piperidino]butyrophenone structure](https://image.chemsrc.com/caspic/109/43076-30-8.png)