TCEP-d16 hydrochloride
Modify Date: 2024-09-23 13:57:33
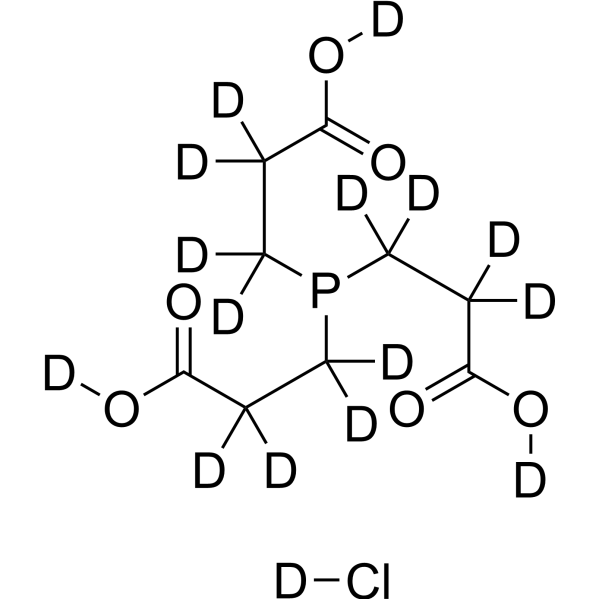
TCEP-d16 hydrochloride structure
|
Common Name | TCEP-d16 hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 1174025-33-2 | Molecular Weight | 302.75 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C9D16ClO6P | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of TCEP-d16 hydrochlorideTCEP-d16 (hydrochloride) is the deuterium labeled TCEP hydrochloride[1]. TCEP hydrochloride (Tris(2-carboxyethyl)phosphine hydrochloride) is a non-thiol reducing agent that is more stable and produces a faster S-S reductive reaction than other chemical reductants. TCEP hydrochloride is a trialkylphosphine, selectively reduces protein disuldes without altering the properties or interacting with thiol-directed agents in the reaction mixture. TCEP hydrochloride is also a commonly used reducing agent in the DNA/AuNP chemistry[2][3][4][5]. |
| Name | TCEP-d16 hydrochloride |
|---|
| Description | TCEP-d16 (hydrochloride) is the deuterium labeled TCEP hydrochloride[1]. TCEP hydrochloride (Tris(2-carboxyethyl)phosphine hydrochloride) is a non-thiol reducing agent that is more stable and produces a faster S-S reductive reaction than other chemical reductants. TCEP hydrochloride is a trialkylphosphine, selectively reduces protein disuldes without altering the properties or interacting with thiol-directed agents in the reaction mixture. TCEP hydrochloride is also a commonly used reducing agent in the DNA/AuNP chemistry[2][3][4][5]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Molecular Formula | C9D16ClO6P |
|---|---|
| Molecular Weight | 302.75 |