calpeptin
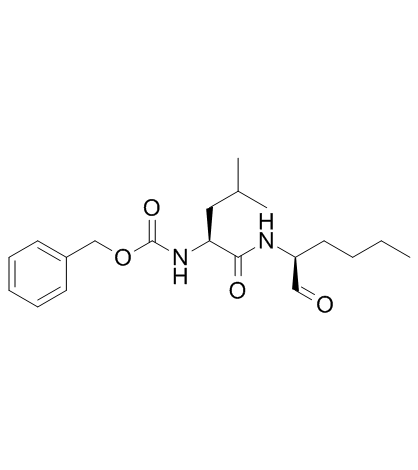
calpeptin structure
|
Common Name | calpeptin | ||
|---|---|---|---|---|
| CAS Number | 117591-20-5 | Molecular Weight | 362.463 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 550.7±45.0 °C at 760 mmHg | |
| Molecular Formula | C20H30N2O4 | Melting Point | 60-75 °C | |
| MSDS | USA | Flash Point | 286.8±28.7 °C | |
Use of calpeptinCalpeptin is a potent, cell penetrating calpain inhibitor, with an ID50 of 40 nM for Calpain I in human platelets[1]. Calpeptin is also an inhibitor of cathepsin K[2]. |
| Name | Calpeptin,N-Benzyloxycarbonyl-L-leucylnorleucinal |
|---|---|
| Synonym | More Synonyms |
| Description | Calpeptin is a potent, cell penetrating calpain inhibitor, with an ID50 of 40 nM for Calpain I in human platelets[1]. Calpeptin is also an inhibitor of cathepsin K[2]. |
|---|---|
| Related Catalog | |
| Target |
ID50: 40 nM (Calpain I in human platelets)[1]. Cthepsin K[2] |
| In Vitro | Calpeptin (0-100 nM, 24 hours) treatment suppresses the proliferation of both WI38 VA13 and IMR90 cells in a dose-dependent manner. Calpeptin (1000 pg/ml, 24 hours) inhibits IL-6-induced cell proliferation of lung fibroblasts[3]. Cell Proliferation Assay[3] Cell Line: WI38 VA13 and IMR90 cells Concentration: 0-100 nM Incubation Time: 24 hours Result: Suppressed the proliferation in a dose-dependent manner. |
| In Vivo | Calpeptin with Bleo (0.04 mg/mouse, i.p., 3 times weekly, 28 days) administration significantly inhibits the collagen deposition and increases of calpain activity in the bleomycintreatedmouse lung tissues[3]. Animal Model: C57BL/6 female mice (Eight-week-old)[3]. Dosage: 0.04 mg/mouse. Administration: Intraperitoneally three times weekly for 28 days (together with Bleo). Result: Inhibited the collagen deposition and increase of calpain activity in the bleomycintreatedmouse lung tissues. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 550.7±45.0 °C at 760 mmHg |
| Melting Point | 60-75 °C |
| Molecular Formula | C20H30N2O4 |
| Molecular Weight | 362.463 |
| Flash Point | 286.8±28.7 °C |
| Exact Mass | 362.220551 |
| PSA | 84.50000 |
| LogP | 4.37 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.508 |
| Storage condition | -20°C |
| Safety Phrases | S24/25 |
|---|---|
| RIDADR | NONH for all modes of transport |
|
~79% 
calpeptin CAS#:117591-20-5 |
| Literature: Tavares, Francis X.; Deaton, David N.; Miller, Aaron B.; Miller, Larry R.; Wright, Lois L. Bioorganic and Medicinal Chemistry Letters, 2005 , vol. 15, # 17 p. 3891 - 3895 |
|
~% 
calpeptin CAS#:117591-20-5 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 15, # 17 p. 3891 - 3895 |
|
~% 
calpeptin CAS#:117591-20-5 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 15, # 17 p. 3891 - 3895 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Calpain mediates processing of the translation termination factor eRF3 into the IAP-binding isoform p-eRF3.
FEBS Lett. 589 , 2241-7, (2015) The involvement of polypeptide chain-releasing factor eRF3 in translation termination and mRNA decay is well established. Moreover, the finding that the proteolytically processed isoform of eRF3 (p-eR... |
|
|
Cytosolic HMGB1 controls the cellular autophagy/apoptosis checkpoint during inflammation.
J. Clin. Invest. 125(3) , 1098-110, (2015) The intracellular protein HMGB1 is released from cells and acts as a damage-associated molecular pattern molecule during many diseases, including inflammatory bowel disease (IBD); however, the intrace... |
|
|
Salbutamol inhibits RhoA activation in normal but not in desensitized bronchial smooth muscle cells.
J. Pharm. Pharmacol. 67 , 1416-20, (2015) This study was aimed at investigating whether the β2 -adrenoceptor agonist, salbutamol, could modulate RhoA activation in normal and homologously desensitized bronchial smooth muscle cells (BSMC).Seru... |
| Z-LEU-NLE-CHO |
| BENZYLOXYCARBONYLLEUCYL-NORLEUCINAL |
| N-Benzyloxycarbonyl-L-leucylnorieucinal Z-Leu-Nle-CHO |
| MFCD00155623 |
| N-CBZ-L-LEUCYL-NORLEUCINAL |
| N-[(benzyloxy)carbonyl]-N-[(2S)-1-oxohexan-2-yl]-L-leucinamide |
| Z-Leu-Nle-H |
| CalpeptinN-Benzyloxycarbonyl-L-leucyl-norleucinal |
| CALPEPTIN |
| Z-LEU-NLE-OH |
| Z-LEU-NORLEUCINAL |
| Carbamic acid, N-[(1S)-1-[[[(1S)-1-formylpentyl]amino]carbonyl]-3-methylbutyl]-, phenylmethyl ester |
| N-BENZYLOXYCARBONYL-L-LEUCYLNORLEUCINAL |
| BENZYLOXYCARBONYLDIPEPTIDYL ALDEHYDE |
| N-benzyloxycarbonyl-L-leucyl-L-norleucinal |
| N-[(Benzyloxy)carbonyl]-N-[(2S)-1-oxo-2-hexanyl]-L-leucinamide |