Dexamethasone-17-acetate

Dexamethasone-17-acetate structure
|
Common Name | Dexamethasone-17-acetate | ||
|---|---|---|---|---|
| CAS Number | 1177-87-3 | Molecular Weight | 434.498 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 579.4±50.0 °C at 760 mmHg | |
| Molecular Formula | C24H31FO6 | Melting Point | 238-240 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 304.2±30.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Dexamethasone-17-acetateDexamethasone acetate is a glucocorticoid receptor agonist. |
| Name | Dexamethasone-17-acetate |
|---|---|
| Synonym | More Synonyms |
| Description | Dexamethasone acetate is a glucocorticoid receptor agonist. |
|---|---|
| Related Catalog | |
| Target |
Glucocorticoid receptor[1] |
| In Vitro | Dexamethasone regulates several transcription factors, including activator protein-1, nuclear factor-AT, and nuclear factor-kB, leading to the activation and repression of key genes involved in the inflammatory response[1]. Dexamethasone potently inhibits granulocyte-macrophage colony stimulating factor (GM-CSF) release from A549 cells with EC50 of 2.2 nM. Dexamethasone (EC50=36 nM) induces transcription of the β2-receptor is found to correlate with glucocorticoid receptor (GR) DNA binding and occurred at 10-100 fold higher concentrations than the inhibition of GM-CSF release. Dexamethasone (IC50=0.5 nM) inhibits a 3×κB (NF-κB, IκBα, and I-κBβ), which is associated with inhibition of GM-CSF release[2]. |
| In Vivo | It has previously been reported that treatment with Dexamethasone at a dose of 2×5 mg/kg efficiently inhibits lipopolysaccharide (LPS)-induced inflammation. In our experimental system, treatment with a single dose of Dexamethasone 10 mg/kg (i.p.) significantly decreases recruitment of granulocytes as well as spontaneous production of oxygen radicals compared with animals expose to LPS and injected with solvent alone (saline). The effects are statistically significant when administered both 1 h before and 1 h after inhalation of LPS. The number of granulocytes in BALF decreased to levels comparable to healthy animals (given an aerosol of water)[3]. Rats treated with Dexamethasone consume less food and weighed less than control rats. Treated rats also weigh less than pair-fed animals though their food intake is similar. Five days of Dexamethasone injection result in a significant increase in both the liver mass (+42%) and the liver to body weight ratio (+65%). The wet weight of gastrocnemius muscle decreases 20% after 5 days of treatment, but it remains unaffected relative to body weight (g/100 g body weight), indicating that muscle weight loss paralleled body weight loss[4]. |
| Animal Admin | Mice[3] Female C57Bl/6JBom mice (age 10-12 weeks) are used in all experiments. Dexamethasone is administered as a single injection of 1 or 10 mg/kg. Dexamethasone is dissolved in saline and 400 μL are injected intraperitoneally, either 1 h before or 1 h after LPS exposure. In one experiment, N-acetylcysteine (NAC) (100 and 500 mg/kg) is injected successively every 4•5 h, starting 1 h before challenge (five injections in total). A control group of LPS-exposed animals are injected intraperitoneally with solvent alone (saline). Intratracheal administration is performed by instillation of 100 μL NAC (50, 100 or 500 mg/kg) or Dexamethasone (10 mg/kg) into the lungs of mice anaesthetized with 15 mg/kg Rapinovet (i.v.). Rats[4] Male Sprague-Dawley rats are used.Dexamethasone-treated rats are injected intraperitoneally once daily with Dexamethasone (1.5 mg/kg body weight) for 5 days and are allowed to feed ad libitum. The Dexamethasone dose (1.5 mg/kg/day) and the duration of treatment (5 days) are specifically chosen as this treatment induced a reproducible and marked catabolic state. Control rats received no treatment and are fed ad libitum. In order to take into account the decrease in food intake induced by Dexamethasone treatment, a third group of pair-fed rats are used. These rats are provided with the same amount of food as Dexamethasone-injected rats and are treated with a daily isovolumic intraperitoneal injection of NaCl (0.9%) for 5 days. After the final injection of Dexamethasone or NaCl, the animals are fasted overnight prior to being killed by decapitation. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 579.4±50.0 °C at 760 mmHg |
| Melting Point | 238-240 °C(lit.) |
| Molecular Formula | C24H31FO6 |
| Molecular Weight | 434.498 |
| Flash Point | 304.2±30.1 °C |
| Exact Mass | 434.210480 |
| PSA | 100.90000 |
| LogP | 2.96 |
| Vapour Pressure | 0.0±3.6 mmHg at 25°C |
| Index of Refraction | 1.571 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H317 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R43 |
| Safety Phrases | S36/37 |
| RIDADR | 2811.0 |
| WGK Germany | 3 |
| RTECS | TU4050000 |
| Hazard Class | 6.1 |
| HS Code | 2937229000 |
| HS Code | 2937229000 |
|---|
|
Upregulation of nucleoside triphosphate diphosphohydrolase-1 and ecto-5'-nucleotidase in rat hippocampus after repeated low-dose dexamethasone administration.
J. Mol. Neurosci. 55(4) , 959-67, (2015) Although dexamethasone (DEX), a synthetic glucocorticoid receptor (GR) analog with profound effects on energy metabolism, immune system, and hypothalamic-pituitary-adrenal axis, is widely used therape... |
|
|
Drug release mechanisms of steroid eluting rings in cardiac pacemaker lead electrodes.
Conf. Proc. IEEE Eng. Med. Biol. Soc. 2012 , 681-4, (2012) This paper reports on the drug release mechanisms of silicone structures with embedded steroids applied in pacing leads. Different derivatives of the steroid dexamethasone, which is associated with th... |
|
|
Intra-articular bioactivity of a p38 MAPK inhibitor and development of an extended-release system.
Eur. J. Pharm. Biopharm. 93 , 110-7, (2015) In the treatment of arthritic diseases, oral or systemic administration of anti-inflammatory substances, such as p38 MAPK inhibitors, is hampered by numerous side effects. To overcome them, formulatio... |
| 9α-Fluoro-11β,17α,21-trihydroxy-16α-methyl-1,4-pregnadiene-3,20-dione 21-Acetate |
| Pregna-1,4-diene-3,20-dione, 9-fluoro-11β,17,21-trihydroxy-16α-methyl-, 21-acetate (8CI) |
| (11β,16α)-9-Fluoro-11,17-dihydroxy-16-methyl-3,20-dioxopregna-1,4-dien-21-yl acetate |
| Pregna-1,4-diene-3,20-dione, 21-(acetyloxy)-9-fluoro-11,17-dihydroxy-16-methyl-, (11β,16α)- |
| MFCD00027407 |
| (11b,16a)-9-Fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione 21-Acetate |
| Dexamethasone acetate |
| EINECS 214-646-8 |
| DEXAMETHASONE 21-ACETATE |
| Dexamethasone (acetate) |
 CAS#:912-38-9
CAS#:912-38-9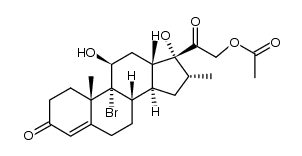 CAS#:34542-57-9
CAS#:34542-57-9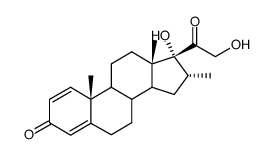 CAS#:19784-87-3
CAS#:19784-87-3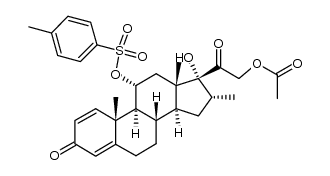 CAS#:115606-30-9
CAS#:115606-30-9 CAS#:78761-59-8
CAS#:78761-59-8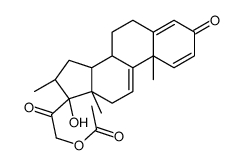 CAS#:10106-41-9
CAS#:10106-41-9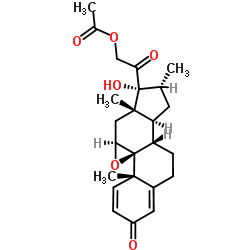 CAS#:2884-51-7
CAS#:2884-51-7 CAS#:50-02-2
CAS#:50-02-2
