donepezil
Modify Date: 2024-01-02 08:26:21
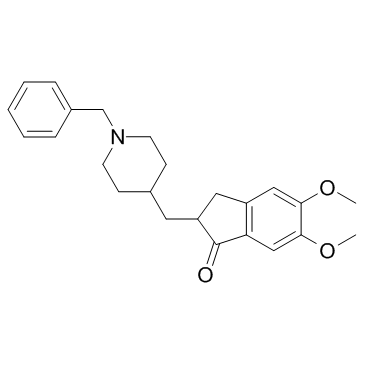
donepezil structure
|
Common Name | donepezil | ||
|---|---|---|---|---|
| CAS Number | 120014-06-4 | Molecular Weight | 379.492 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 527.9±50.0 °C at 760 mmHg | |
| Molecular Formula | C24H29NO3 | Melting Point | 207ºC | |
| MSDS | N/A | Flash Point | 273.1±30.1 °C | |
Use of donepezilDonepezil(E 2020) is a specific and potent AChE inhibitor for bAChE and hAChE with IC50 of 8.12 nM and 11.6 nM, respectively.Target: AChEDonepezil is a specific and potent AChE inhibitor for bAChE and hAChE with IC50 of 8.12 nM and 11.6 nM , respectively [1]. Donepezil inhibits the carbachol-stimulated increase in intracellular Ca2+ concentration in human SHSY5Y neuroblastoma cells in a concentration dependent manner, indicating that Donepezil have muscarinic antagonist activity. Intraperitoneal administration of Donepezil in rats produces a dose dependent increase in salivation and tremor, which are overt cholinergic behavioural signs, with an ED50 of 6 μmol/kg. Donepezil is found to be somewhat less potent with a ED50 of 50 μmol/kg following oral administration [2]. A recent study shows that Donepezil can protect human umbilical vein endothelial cells (HUVECs) against H2O2-induced cell injury. This may be useful as a potential therapy for oxidative stress in cardiovascular and cerebrovascular diseases [3]. |
| Name | donepezil |
|---|---|
| Synonym | More Synonyms |
| Description | Donepezil(E 2020) is a specific and potent AChE inhibitor for bAChE and hAChE with IC50 of 8.12 nM and 11.6 nM, respectively.Target: AChEDonepezil is a specific and potent AChE inhibitor for bAChE and hAChE with IC50 of 8.12 nM and 11.6 nM , respectively [1]. Donepezil inhibits the carbachol-stimulated increase in intracellular Ca2+ concentration in human SHSY5Y neuroblastoma cells in a concentration dependent manner, indicating that Donepezil have muscarinic antagonist activity. Intraperitoneal administration of Donepezil in rats produces a dose dependent increase in salivation and tremor, which are overt cholinergic behavioural signs, with an ED50 of 6 μmol/kg. Donepezil is found to be somewhat less potent with a ED50 of 50 μmol/kg following oral administration [2]. A recent study shows that Donepezil can protect human umbilical vein endothelial cells (HUVECs) against H2O2-induced cell injury. This may be useful as a potential therapy for oxidative stress in cardiovascular and cerebrovascular diseases [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 527.9±50.0 °C at 760 mmHg |
| Melting Point | 207ºC |
| Molecular Formula | C24H29NO3 |
| Molecular Weight | 379.492 |
| Flash Point | 273.1±30.1 °C |
| Exact Mass | 379.214752 |
| PSA | 38.77000 |
| LogP | 4.71 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.578 |
| Water Solubility | 2.931 mg/L |
| Hazard Codes | Xi: Irritant; |
|---|---|
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | UN 2811 |
| Precursor 10 | |
|---|---|
| DownStream 4 | |
| 2-((1-Benzylpiperidin-4-yl)methyl)-5,6-dimethoxy-2,3-dihydro-1H-inden-1-one |
| 2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimethoxy-2,3-dihydro-1H-inden-1-one |
| 2,3-Dihydro-5,6-dimethoxy-2-[[1-(phenylmethyl)-4-piperidinyl]methyl]-1H-inden-1-one |
| 5,6-Dimethoxy-2-[[1-(phenylmethyl)-4-piperidinyl]methyl]-2,3-dihydro-1H-inden-1-one |
| n-1-one |
| 2-[(1-Benzyl-4-piperidyl)methyl]-5,6-dimethoxy-2,3-dihydroinden-1-one |
| 1H-Inden-1-one, 2,3-dihydro-5,6-dimethoxy-2-[[1-(phenylmethyl)-4-piperidinyl]methyl]- |
| Aricept |
| Donepezil hydrochloride monohydrate |
| (±)-E-2020 |
| 2-[(1-Benzyl-4-piperidinyl)methyl]-5,6-dimethoxy-1-indanone |
| 2-[(1-Benzylpiperidin-4-yl)methyl]-5,6-dimethoxyindan-1-one |
| MFCD00912833 |
| donepezil |
| 1-BENZYL-4-[(5,6-DIMETHOXY-1-INDANON-2-YL)METHYL]PIPERIDINE |
| 5,6-Dimethoxy-2,3-dihydro-1H-inden-1-one |
| Donezepil |
| 2-[(1-Benzylpiperidin-4-yl)methyl]-5,6-dimethoxy-2,3-dihydro-1H-inden-1-on |
| Donepezil Base |
 CAS#:100-39-0
CAS#:100-39-0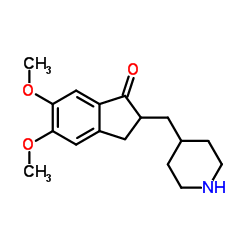 CAS#:120014-30-4
CAS#:120014-30-4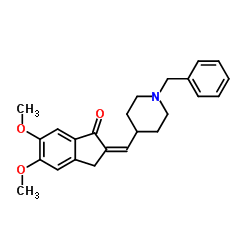 CAS#:120014-07-5
CAS#:120014-07-5![2-[(1-Benzylpiperidin-4-yl)hydroxyMethyl]-5,6-dimethoxyindan-1-one Structure](https://image.chemsrc.com/caspic/159/197010-20-1.png) CAS#:197010-20-1
CAS#:197010-20-1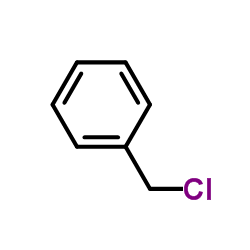 CAS#:100-44-7
CAS#:100-44-7![1-[(1-benzoylpiperidin-4-yl)methyl]-5,6-dimethoxyindan-1-one Structure](https://image.chemsrc.com/caspic/487/120013-38-9.png) CAS#:120013-38-9
CAS#:120013-38-9![5-[(1-benzyl-4-piperidyl)methyl]-5-(3,4-dimethoxybenzyl)-2,2-dimethyl-1,3-dioxane-4,6-dione Structure](https://image.chemsrc.com/caspic/324/848610-96-8.png) CAS#:848610-96-8
CAS#:848610-96-8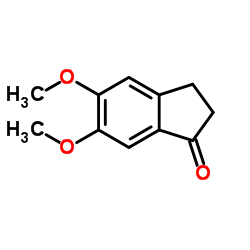 CAS#:2107-69-9
CAS#:2107-69-9 CAS#:22065-85-6
CAS#:22065-85-6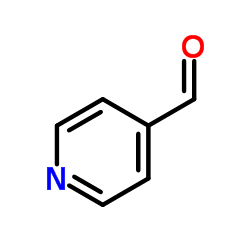 CAS#:872-85-5
CAS#:872-85-5 CAS#:120013-56-1
CAS#:120013-56-1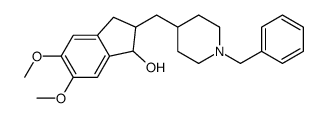 CAS#:120012-04-6
CAS#:120012-04-6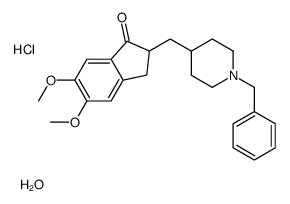 CAS#:884740-09-4
CAS#:884740-09-4
