Rifalazil
Modify Date: 2024-01-10 12:05:41
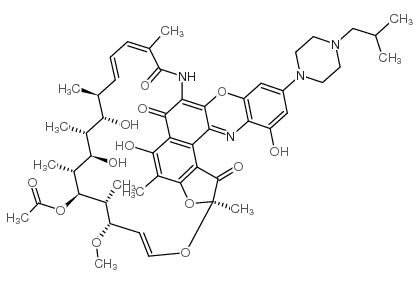
Rifalazil structure
|
Common Name | Rifalazil | ||
|---|---|---|---|---|
| CAS Number | 129791-92-0 | Molecular Weight | 941.07300 | |
| Density | 1.36g/cm3 | Boiling Point | 1048.6ºC at 760mmHg | |
| Molecular Formula | C51H64N4O13 | Melting Point | 195-200° (dec) | |
| MSDS | N/A | Flash Point | 588ºC | |
Use of RifalazilRifalazil (KRM-1648; ABI-1648), a rifamycin derivative, inhibits the bacterial DNA-dependent RNA polymerase and kills bacterial cells by blocking off the β-subunit in RNA polymerase[1]. Rifalazil (KRM-1648; ABI-1648) is an antibiotic, exhibits high potency against mycobacteria, gram-positive bacteria, Helicobacter pylori, C. pneumoniae and C. trachomatis with MIC values from 0.00025 to 0.0025 μg/ml[3]. Rifalazil (KRM-1648; ABI-1648) has the potential for the treatment of Chlamydia infection, Clostridium difficile associated diarrhea (CDAD), and tuberculosis (TB)[2]. |
| Name | Rifalazil |
|---|---|
| Synonym | More Synonyms |
| Description | Rifalazil (KRM-1648; ABI-1648), a rifamycin derivative, inhibits the bacterial DNA-dependent RNA polymerase and kills bacterial cells by blocking off the β-subunit in RNA polymerase[1]. Rifalazil (KRM-1648; ABI-1648) is an antibiotic, exhibits high potency against mycobacteria, gram-positive bacteria, Helicobacter pylori, C. pneumoniae and C. trachomatis with MIC values from 0.00025 to 0.0025 μg/ml[3]. Rifalazil (KRM-1648; ABI-1648) has the potential for the treatment of Chlamydia infection, Clostridium difficile associated diarrhea (CDAD), and tuberculosis (TB)[2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: RNA polymerase[1] |
| In Vitro | Rifalazil exhibits antimicrobal activity against Gram-positive enteric bacteria, inhibits Clostridium difficile, Clostridium perfringens, Bacteroides fragilis with MIC50 value of 0.0015, 0.0039, 0.0313 µg/ml, respectively[3]. Rifalazil exhibits antimicrobal activity against Gram-negative enteric bacteria, inhibits Escherichia coli and Klebsiella pneumoniae with MIC50 value of 16 and 16 µg/ml, respectively[3]. Rifalazil exhibits antimicrobal activity against non-enteric Gram-positive bacteria, inhibits Methicillin-susceptible Staphylococcus aureus, Methicillin-resistant S. aureus, Methicillin- and quinolone-resistant S. aureus, Staphylococcus epidermidis, Streptococcus pyogenes, Streptococcus pneumoniae with MIC50 value of 0.0078, 0.0078, 0.0078, 0.0078, 0.0002, 0.0001 µg/ml, respectively[3]. Rifalazil exhibits antimicrobal activity against Helicobacter pylori, Chlamydia pneumoniae and Chlamydia trachomatis with MIC50 value of 0.004, 0.000125 and 0.00025 µg/ml, respectively[3]. |
| In Vivo | Rifalazil (oral gavage; 20, 25, and 150 mg/kg; 6-8 weeks) combines with isoniazid (INH) for 6 weeks or greater significantly reduced the number of mice per group in which M. tuberculosis is detected in both spleens and lungs compared to the reductions for the early and late controls. And the addition of Pyrazinamide (PZA) does not significantly improve RLZ-INH therapy at any time point[2]. Animal Model: Female CD-1 mice infected with 5.2 × 107 viable mycobacteria[2] Dosage: 20, 25, and 150 mg/kg; 6-8 weeks Administration: Oral gavage Result: Combined with isoniazid (INH) showed its potential for short-course treatment of Mycobacterium tuberculosis infection. |
| References |
| Density | 1.36g/cm3 |
|---|---|
| Boiling Point | 1048.6ºC at 760mmHg |
| Melting Point | 195-200° (dec) |
| Molecular Formula | C51H64N4O13 |
| Molecular Weight | 941.07300 |
| Flash Point | 588ºC |
| Exact Mass | 940.44700 |
| PSA | 230.66000 |
| LogP | 6.57360 |
| Vapour Pressure | 0mmHg at 25°C |
| Index of Refraction | 1.634 |
| krm 1648 |