(-)-Catechin gallate(CG)
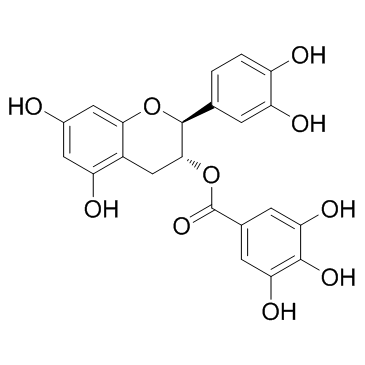
(-)-Catechin gallate(CG) structure
|
Common Name | (-)-Catechin gallate(CG) | ||
|---|---|---|---|---|
| CAS Number | 130405-40-2 | Molecular Weight | 442.372 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | 861.7±65.0 °C at 760 mmHg | |
| Molecular Formula | C22H18O10 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 305.0±27.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of (-)-Catechin gallate(CG)(-)-Catechin gallate is a minor constituent in green tea catechins. (-)-Catechin gallate inhibits the activity of COX-1 and COX-2 enzymes. |
| Name | (-)-Catechin Gallate |
|---|---|
| Synonym | More Synonyms |
| Description | (-)-Catechin gallate is a minor constituent in green tea catechins. (-)-Catechin gallate inhibits the activity of COX-1 and COX-2 enzymes. |
|---|---|
| Related Catalog | |
| Target |
COX-1 COX-2 |
| In Vitro | (-)-Catechin gallate (CG) directly interacts with DNA oligomers and inhibits the activity of COX-1 and COX-2 enzymes, the gene expression of matrix metalloproteinase-9 in macrophage-differentiated HL-60 myeloid leukemia cells, the adipocyte uptake of glucose by the transporter, GLUT4, and the activities of various proteasomes, i.e., the multicatalytic proteases responsible for the degradation of most cellular proteins. The relative cytotoxicities of a 3-day exposure to (-)-Catechin gallate are determined for cancerous CAL27 and HSG cells, immortalized epithelioid S-G cells, and normal HGF-1 gingival fibroblasts. The concentration at which toxicity (P≤0.01) initially occur is 25 μM (-)-Catechin gallate for S-G cells, 50 μM (-)-Catechin gallate for CAL27 cells, 62.5 μM (-)-Catechin gallate for HSG cells and 75 μM (-)-Catechin gallate for HGF-1 fibroblasts. The calculated neutral red (NR50) values for a 3-day exposure to (-)-Catechin gallate are 58 μM for S-G cells, 62 μM for CAL27 cells, 90 μM for HSG cells and 132 μM for HGF-1 fibroblasts[1]. |
| Cell Assay | Human tongue squamous carcinoma (CAL27) cells and human salivary gland carcinoma (HSG) cells are used. Individual wells of a 96-well microtiter tissue culture plate are inoculated with 0.2 mL of the growth medium containing 2×104 cells/well for a 1-day exposure, 1.5×104 cells/ well for a 2-day exposure and 1×104 cells/well for a 3-day exposure to the test agents. After 1 day of incubation, the growth medium is removed and replaced with exposure medium, with or without varied concentrations of the test agents. In some studies the cells are coexposed to (-)-Catechin gallate (100, 200, 300, 400, and 500 μM) and 100 Units/mL catalase. After 1-3 days of exposure to the test agents, viability is assessed with the neutral red (NR) assay, which is based on the uptake and accumulation of the supravital dye, neutral red (NR)[1]. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 861.7±65.0 °C at 760 mmHg |
| Molecular Formula | C22H18O10 |
| Molecular Weight | 442.372 |
| Flash Point | 305.0±27.8 °C |
| Exact Mass | 442.089996 |
| PSA | 177.14000 |
| LogP | 2.67 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.825 |
| Storage condition | 2~8℃ |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
|
Development and validation of UHPLC-MS/MS method for determination of eight naturally occurring catechin derivatives in various tea samples and the role of matrix effects.
J. Pharm. Biomed. Anal. 114 , 62-70, (2015) A complete analytical procedure combining optimized tea infusion preparation and validated UHPLC-MS/MS method was developed for routine quantification of eight naturally occurring catechin derivatives... |
|
|
Catechin gallates are NADP+-competitive inhibitors of glucose-6-phosphate dehydrogenase and other enzymes that employ NADP+ as a coenzyme.
Bioorg. Med. Chem. 16 , 3580-6, (2008) Recent studies have shown that glucose-6-phosphate dehydrogenase (G6PD) is an effectual therapeutic target for metabolic disorders, including obesity and diabetes. In this study, we used in silico and... |
|
|
Flavonoids as opioid receptor ligands: identification and preliminary structure-activity relationships.
J. Nat. Prod. 70 , 1278-82, (2007) Flavonoids have been recognized as the active ingredients of many medicinal plant extracts due to interactions with proteins via phenolic groups and low toxicity. Here, we report the investigation of ... |
| (−)-Catechin gallate |
| (2S,3R)-2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-3,4-dihydro-2H-chromen-3-yl 3,4,5-trihydroxybenzoate |
| MFCD00214258 |
| (2S,3R)-2-(3,4-Dihydroxyphenyl)-5,7-dihydroxychroman-3-yl 3,4,5-trihydroxybenzoate |
| [(2S,3R)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3,4-dihydro-2H-chromen-3-yl] 3,4,5-trihydroxybenzoate |
| Benzoic acid, 3,4,5-trihydroxy-, (2S,3R)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-5,7-dihydroxy-2H-1-benzopyran-3-yl ester |
![(-)-(2S,3R)-5,7-bis(benzyloxy)-2-[3,4-bis(benzyloxy)phenyl]chroman-3-yl 3,4,5-tris(benzyloxy)benzoate Structure](https://image.chemsrc.com/caspic/293/732298-10-1.png) CAS#:732298-10-1
CAS#:732298-10-1 CAS#:63604-98-8
CAS#:63604-98-8 CAS#:1486-48-2
CAS#:1486-48-2 CAS#:1486-47-1
CAS#:1486-47-1![(E)-3-[2,4-bis(benzyloxy)-6-hydroxyphenyl]-1-[3,4-bis(benzyloxy)phenyl]propene Structure](https://image.chemsrc.com/caspic/196/732298-08-7.png) CAS#:732298-08-7
CAS#:732298-08-7![(-)-(2S,3R)-5,7-bis(benzyloxy)-2-[3,4-bis(benzyloxy)phenyl]chroman-3-ol Structure](https://image.chemsrc.com/caspic/239/87292-50-0.png) CAS#:87292-50-0
CAS#:87292-50-0![(-)-(1R,2R)-3-[2,4-bis(benzyloxy)-6-hydroxyphenyl]-1-[3,4-bis(benzyloxy)phenyl]propane-1,2-diol Structure](https://image.chemsrc.com/caspic/158/732298-09-8.png) CAS#:732298-09-8
CAS#:732298-09-8 CAS#:1257-08-5
CAS#:1257-08-5
