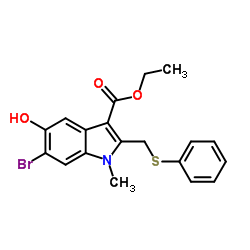
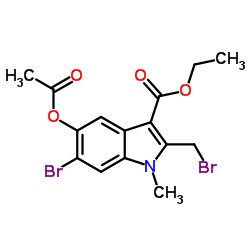
Arbidol
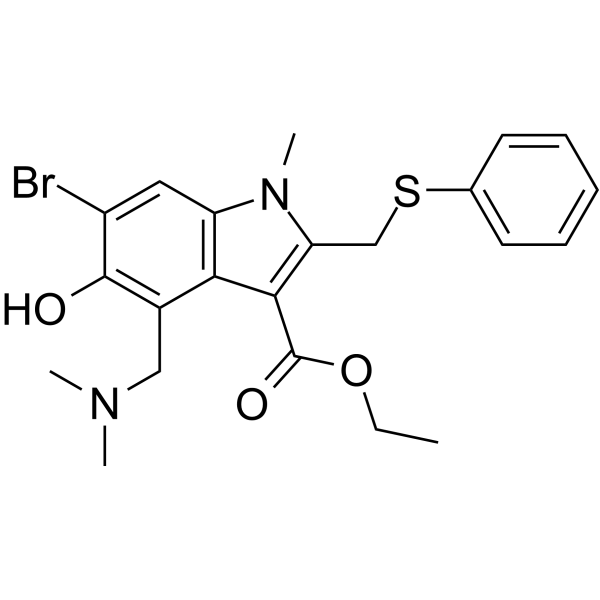
Arbidol structure
|
Common Name | Arbidol | ||
|---|---|---|---|---|
| CAS Number | 131707-25-0 | Molecular Weight | 477.41 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 591.8±50.0 °C at 760 mmHg | |
| Molecular Formula | C22H25BrN2O3S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 311.7±30.1 °C | |
Use of ArbidolUmifenovir is a potent, orally active broad-spectrum antiviral agent with activity against a number of enveloped and non-enveloped viruses. Umifenovir is used as an anti-influenza virus agent. Umifenovir could effectively inhibit the fusion of virus with host cells[1][2]. Umifenovir is an efficient inhibitor of SARS-CoV-2 in vitro[2]. Umifenovir shows anti-inflammatory activity[3]. |
| Name | ethyl 6-bromo-4-[(dimethylamino)methyl]-5-hydroxy-1-methyl-2-(phenylsulfanylmethyl)indole-3-carboxylate |
|---|---|
| Synonym | More Synonyms |
| Description | Umifenovir is a potent, orally active broad-spectrum antiviral agent with activity against a number of enveloped and non-enveloped viruses. Umifenovir is used as an anti-influenza virus agent. Umifenovir could effectively inhibit the fusion of virus with host cells[1][2]. Umifenovir is an efficient inhibitor of SARS-CoV-2 in vitro[2]. Umifenovir shows anti-inflammatory activity[3]. |
|---|---|
| Related Catalog | |
| In Vitro | Umifenovir exhibits a wide range and potent antiviral activity against a number of viruses including influenza viruses A, B and C, respiratory syncytial virus, SARS-CoV, adenovirus, parainfluenza type 5, poliovirus 1, rhinovirus 14, coxsackievirus B5, hantaan virus, Chikungunya virus, HBV and HCV[1]. |
| In Vivo | Umifenovir (25 and 45 mg/ml; p.o.;) shows a survival benefit to mice suffering from influenza infection[3]. Animal Model: BALB/c mice (6–8 weeks old), mice were intranasally (i.n.) inoculated with 2 times the 50% mouse lethal dose (MLD50) of A/ Guangdong/GIRD07/09 (H1N1) (104.5TCID50/mL) in a volume of 20mL[3]. Dosage: 1.25 mg/mL (25 mg/kg/day) and 2.25 mg/mL (45 mg/kg/day) in a total volume of 500mL at one day pre-infection and 3 days post-infection (dpi) Administration: Oral administration Result: Increased the survival rate, inhibited the decrease of body weight at 45 mg/mL and inhibited the increase of mice lung index at 25 mg/mL and 45 mg/mL comparing to virus group. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 591.8±50.0 °C at 760 mmHg |
| Molecular Formula | C22H25BrN2O3S |
| Molecular Weight | 477.41 |
| Flash Point | 311.7±30.1 °C |
| Exact Mass | 476.076904 |
| PSA | 80.00000 |
| LogP | 4.64 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.620 |
|
~86% 
Arbidol CAS#:131707-25-0 |
| Literature: Pharmaceutical Chemistry Journal, , vol. 27, # 1 p. 75 - 76 Khimiko-Farmatsevticheskii Zhurnal, , vol. 27, # 1 p. 70 - 71 |
|
~% 
Arbidol CAS#:131707-25-0 |
| Literature: US5198552 A1, ; |
|
~71% 
Arbidol CAS#:131707-25-0 |
| Literature: Pharmaceutical Chemistry Journal, , vol. 27, # 1 p. 75 - 76 Khimiko-Farmatsevticheskii Zhurnal, , vol. 27, # 1 p. 70 - 71 |
|
~% 
Arbidol CAS#:131707-25-0 |
| Literature: Pharmaceutical Chemistry Journal, , vol. 27, # 1 p. 75 - 76 Khimiko-Farmatsevticheskii Zhurnal, , vol. 27, # 1 p. 70 - 71 |
|
~% 
Arbidol CAS#:131707-25-0 |
| Literature: Pharmaceutical Chemistry Journal, , vol. 27, # 1 p. 75 - 76 Khimiko-Farmatsevticheskii Zhurnal, , vol. 27, # 1 p. 70 - 71 |
| 1H-Indole-3-carboxylic acid, 6-bromo-4-[(dimethylamino)methyl]-5-hydroxy-1-methyl-2-[(phenylthio)methyl]-, ethyl ester |
| 1-methyl-2-phenylthiomethyl-3-ethoxycarbonyl-4-dimethylaminomethyl-5-hydroxy-6-bromindole |
| ethyl 6-bromo-5-hydroxy-4-dimethylaminomethyl-1-methyl-2-phenylthiomethylindole-3-carboxylate |
| Ethyl 6-bromo-4-[(dimethylamino)methyl]-5-hydroxy-1-methyl-2-[(phenylsulfanyl)methyl]-1H-indole-3-carboxylate |
| Umifenovir |
| 6-bromo-5-hydroxy-4-dimethylaminomethyl-1-methyl-2-phenylthiomethylindole-3-carboxylic acid ethyl ester |
| 6-bromo-4-dimethylaminomethyl-3-ethoxycarbonyl-5-hydroxy-1-methyl-2-phenylthiomethylindole |
| Arbidol |
| 1-methyl-2-phenylthiomethyl-3-carbethoxy-4-dimethylaminomethyl-5-oxy-6-bromoindole |
| HMS589B06 |