Pronethalol-d6
Modify Date: 2024-01-10 16:46:16
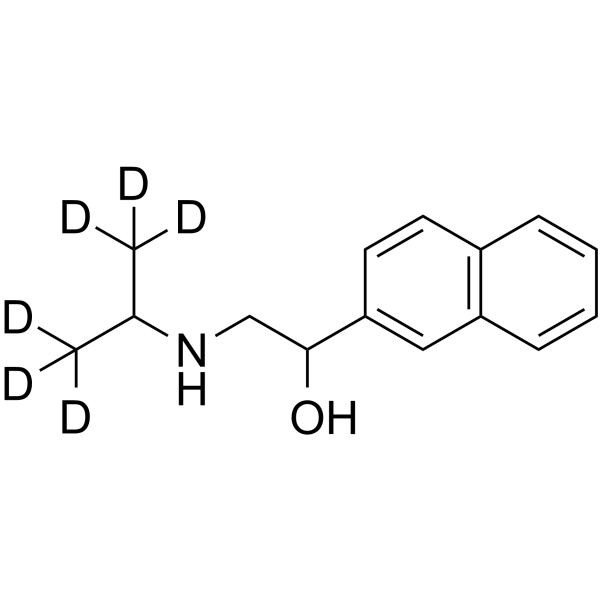
Pronethalol-d6 structure
|
Common Name | Pronethalol-d6 | ||
|---|---|---|---|---|
| CAS Number | 1329805-79-9 | Molecular Weight | 235.35 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C15H13D6NO | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Pronethalol-d6Pronethalol-d6 ((±)-Pronethalo-d6) is the deuterium labeled Pronethalol. Pronethalol ((±)-Pronethalo) is a non-selective β-adrenergic antagonist. Pronethalol is a potent inhibitor of Sox2 expression. Pronethalol protects against and to reverse Digitalis-induced ventricular arrhythmias and limits the cerebral arteriovenous malformation (AVMs)[1][2][3]. |
| Name | Pronethalol-d6 |
|---|
| Description | Pronethalol-d6 ((±)-Pronethalo-d6) is the deuterium labeled Pronethalol. Pronethalol ((±)-Pronethalo) is a non-selective β-adrenergic antagonist. Pronethalol is a potent inhibitor of Sox2 expression. Pronethalol protects against and to reverse Digitalis-induced ventricular arrhythmias and limits the cerebral arteriovenous malformation (AVMs)[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Molecular Formula | C15H13D6NO |
|---|---|
| Molecular Weight | 235.35 |