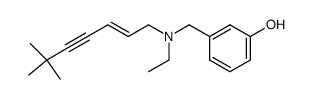
NB598 hydrochloride
Modify Date: 2024-01-20 11:21:35
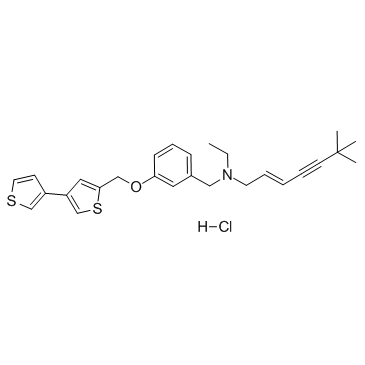
NB598 hydrochloride structure
|
Common Name | NB598 hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 136719-25-0 | Molecular Weight | 486.132 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C27H32ClNOS2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of NB598 hydrochlorideNB-598 hydrochloride is a potent and competitive inhibitor of squalene epoxidase (SE), and suppresses triglyceride biosynthesis through the farnesol pathway. |
| Name | (E)-N-ethyl-6,6-dimethyl-N-[[3-[(4-thiophen-3-ylthiophen-2-yl)methoxy]phenyl]methyl]hept-2-en-4-yn-1-amine,hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | NB-598 hydrochloride is a potent and competitive inhibitor of squalene epoxidase (SE), and suppresses triglyceride biosynthesis through the farnesol pathway. |
|---|---|
| Related Catalog | |
| Target |
squalene epoxidase |
| In Vitro | NB598 (10 μM) causes a 36±7% reduction in total cholesterol level of MIN6 cells. NB598 causes a significant decrease in cholesterol by 49±2%, 46±7%, and 48±2% from PM, ER, and SG, respectively. NB598 dose-dependently inhibits insulin secretion under both basal (1 mM glucose) and glucose-stimulated (16.7 mM glucose) conditions. NB598 at concentrations up to 10 μM does not affect peak outward KV currents or the voltage dependence of activation but increases current inactivation[1]. NB-598 (10 μM) inhibits the synthesis of sterol and sterol ester from [14C]acetate without affecting the synthesis of other lipids such as phospholipids (PL), free fatty acids (FFA) and triacylglycerol (TG). In the absence of exogenous liposomal cholesterol, NB-598 reduces ACAT activity by 31%. NB-598 reduces ACAT activity by 22% even in the presence of a 600 PM concentration of liposomal cholesterol[2]. NB-598 suppresses the secretion of cholesterol and triacylglycerol from HepG2 cells into the medium[3]. |
| Kinase Assay | Caco-2 cells are grown in a 58 cm2 plastic dish with medium A for 13 days. The cells are washed with medium B, and then cultured with medium B including cholesterol-micelle and each compound. The compound is dissolved in Me2SO, and the final concentration of Me2SO is 0.1%(v/v). After 18 hr of incubation, the cells are washed extensively with phosphate-buffered saline (PBS) to remove the compound. Microsomes are prepared as described above. The reaction mixture (0.2 mL) consisted of 0.1 mg microsomes, 0.25% BSA and 40 PM [14C]oleoyl CoA in buffer A. To avoid the effects of endogenous cholesterol, liposome (2 mol of cholesterol: 1 mol of phosphatidylcholine) [15] is added to the reaction mixture. The microsomes are preincubated for 1 hr with or without exogenous cholesterol, and ACAT activity is determined as described above. |
| References |
| Molecular Formula | C27H32ClNOS2 |
|---|---|
| Molecular Weight | 486.132 |
| Exact Mass | 485.161377 |
| PSA | 68.95000 |
| LogP | 8.28520 |
| Storage condition | 2-8℃ |
|
~% 
NB598 hydrochloride CAS#:136719-25-0 |
| Literature: Banyu Pharmaceutical Co., Ltd. Patent: US5234946 A1, 1993 ; |
| (E)-N-(6-6-dimethyl-2-hepten-4-ynyl)-N-ethyl-3-[4-(3-thienyl)-2-thienyl-methyloxy]benzylamine hydrochloride |
| (E)-N-ethyl-N-(6,6-dimethyl-2-heptyn-4-ynyl)-3-[(3,3'-bithiophen-5-yl)methoxy]benzenemethanamine hydrochloride |
| Benzenemethanamine, 3-([3,3'-bithiophen]-5-ylmethoxy)-N-[(2E)-6,6-dimethyl-2-hepten-4-yn-1-yl]-N-ethyl-, hydrochloride (1:1) |
| NB598 hydrochloride |
| (2E)-N-[3-(3,3'-Bithiophen-5-ylmethoxy)benzyl]-N-ethyl-6,6-dimethyl-2-hepten-4-yn-1-amine hydrochloride (1:1) |
| CS-1275 |
| NB-598 hydrochloride||NB598 hydrochloride |
| (E)-N-(6,6-dimethyl-2-hepten-4-ynyl)-N-ethyl-3-[4-(thien-3-yl)-thien-2-yl-methyloxy]benzylamine hydrochloride |
| NB-598 (hydrochloride) |