Agomelatine
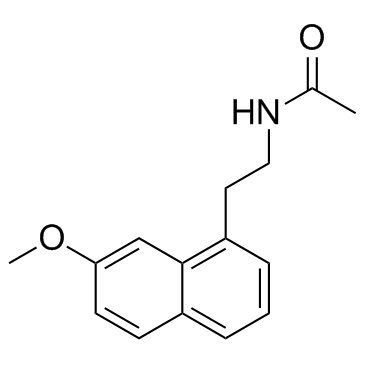
Agomelatine structure
|
Common Name | Agomelatine | ||
|---|---|---|---|---|
| CAS Number | 138112-76-2 | Molecular Weight | 243.301 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 478.8±28.0 °C at 760 mmHg | |
| Molecular Formula | C15H17NO2 | Melting Point | 107-109ºC | |
| MSDS | Chinese USA | Flash Point | 243.4±24.0 °C | |
| Symbol |

GHS09 |
Signal Word | Warning | |
Use of AgomelatineAgomelatine is a competitive antagonist of human and porcine serotonin (5-HT2C) receptors (pKi = 6.2 and 6.4, respectively) as well as human 5-HT2B receptors (pKi = 6.6). IC50 value: 6.2 (pKi, 5-HT2c); 6.6 (pKi, 5-HT2b)Target: 5-HT2C Receptor; 5-HT2B receptorIt is classified as a norepinephrine-dopamine disinhibitor (NDDI) due to its antagonism of the 5-HT2C receptor. Activation of 5-HT2C receptors by serotonin inhibits dopamine and norepinephrine release. Antagonism of 5-HT2C results in an enhancement of DA and NE release and activity of frontocortical dopaminergic and adrenergic pathways. Agomelatine is also a potent agonist at melatonin receptors which makes it the first melatonergic antidepressant. Agomelatine is an antidepressant drug. |
| Name | Agomelatine |
|---|---|
| Synonym | More Synonyms |
| Description | Agomelatine is a competitive antagonist of human and porcine serotonin (5-HT2C) receptors (pKi = 6.2 and 6.4, respectively) as well as human 5-HT2B receptors (pKi = 6.6). IC50 value: 6.2 (pKi, 5-HT2c); 6.6 (pKi, 5-HT2b)Target: 5-HT2C Receptor; 5-HT2B receptorIt is classified as a norepinephrine-dopamine disinhibitor (NDDI) due to its antagonism of the 5-HT2C receptor. Activation of 5-HT2C receptors by serotonin inhibits dopamine and norepinephrine release. Antagonism of 5-HT2C results in an enhancement of DA and NE release and activity of frontocortical dopaminergic and adrenergic pathways. Agomelatine is also a potent agonist at melatonin receptors which makes it the first melatonergic antidepressant. Agomelatine is an antidepressant drug. |
|---|---|
| Related Catalog | |
| References |
[5]. Agomelatine |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 478.8±28.0 °C at 760 mmHg |
| Melting Point | 107-109ºC |
| Molecular Formula | C15H17NO2 |
| Molecular Weight | 243.301 |
| Flash Point | 243.4±24.0 °C |
| Exact Mass | 243.125931 |
| PSA | 38.33000 |
| LogP | 2.27 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.582 |
| Storage condition | -20°C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Precursor 8 | |
|---|---|
| DownStream 2 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
[The activity of glutathione antioxidant system at melaksen and valdoxan action under experimental hyperthyroidism in rats].
Biomed. Khim. 59(5) , 541-9, (2013) Investigation of glutathione antioxidant system activity and diene conjugates content in rats liver and blood serum at the influence of melaksen and valdoxan under experimental hyperthyroidism (EG) ha... |
|
|
[Tachycardia and precordial pain with agomelatine: a case report].
Therapie. 68(5) , 324-5, (2013)
|
|
|
Agomelatine, melatonin and depressive disorder.
Expert Opin. Investig. Drugs 22(4) , 407-10, (2013) Alteration of nocturnal melatonin production, along with circadian rhythm disturbance, has been demonstrated in several psychiatric disorders. It has been postulated that such disturbances might be ca... |
| Unii-137R1N49ad |
| N-[2-(7-Methoxy-1-naphthyl)ethyl]acetamide |
| Acetamide, N-[2-(7-methoxy-1-naphthalenyl)ethyl]- |
| Aglomelatine |
| Agomelatine |
| N-[2-(7-Methoxy-1-naphthalenyl)ethyl]-acetamide S-20098 |
| AGOMELATIN |
| Valdoxan |
| N-(2-(7-Methoxynaphthalen-1-yl)ethyl)acetamide |
| N-[2-(7-Methoxy-1-naphthalenyl)ethyl]acetamide |
| Melitor |
| Thymanax |
 CAS#:108-24-7
CAS#:108-24-7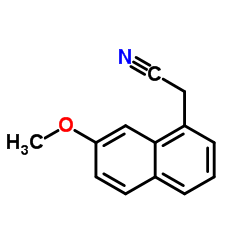 CAS#:138113-08-3
CAS#:138113-08-3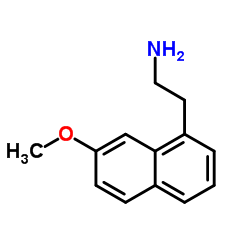 CAS#:138113-09-4
CAS#:138113-09-4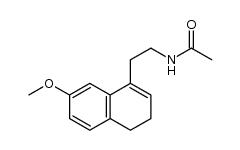 CAS#:1352139-51-5
CAS#:1352139-51-5 CAS#:139525-77-2
CAS#:139525-77-2 CAS#:75-36-5
CAS#:75-36-5 CAS#:77-78-1
CAS#:77-78-1 CAS#:152302-45-9
CAS#:152302-45-9![N-[2-(3-bromo-7-methoxynaphthalen-1-yl)ethyl]acetamide structure](https://image.chemsrc.com/caspic/470/686319-38-0.png) CAS#:686319-38-0
CAS#:686319-38-0
