CAY10574
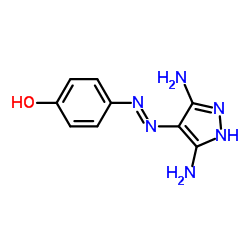
CAY10574 structure
|
Common Name | CAY10574 | ||
|---|---|---|---|---|
| CAS Number | 140651-18-9 | Molecular Weight | 218.215 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 638.3±55.0 °C at 760 mmHg | |
| Molecular Formula | C9H10N6O | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 339.8±31.5 °C | |
Use of CAY10574CAN508 is a potent, ATP-competitive CDK9/cyclin T1 inhibitor with an IC50 of 0.35 μM. CAN508 exhibits a 38-fold selectivity for CDK9/cyclin T over other CDK/cyclin complexes. Antitumor activity[1][2]. |
| Name | 4-[(3,5-diamino-1H-pyrazol-4-yl)hydrazinylidene]cyclohexa-2,5-dien-1-one |
|---|---|
| Synonym | More Synonyms |
| Description | CAN508 is a potent, ATP-competitive CDK9/cyclin T1 inhibitor with an IC50 of 0.35 μM. CAN508 exhibits a 38-fold selectivity for CDK9/cyclin T over other CDK/cyclin complexes. Antitumor activity[1][2]. |
|---|---|
| Related Catalog | |
| Target |
CDK9/cyclinT1:0.35 μM (IC50) CDK2/cyclinE:20 μM (IC50) cdk2/cyclin A:69 μM (IC50) Cdk4/cyclin D1:13.5 μM (IC50) CDK7/cyclin H:26 μM (IC50) Cdk1/cyclin B:44 μM (IC50) |
| In Vitro | CAN508 reduces the frequency of S-phase cells of the cancer cell line HT-29 in antiproliferation assays[1]. CAN508 (20-40 μM; 72 hours) significantly reduces cell proliferation in a dose dependent manner in all three esophageal adenocarcinoma cell lines (SKGT4, OE33 and FLO-1 cells) with IC50s ranging from 34.99 to 91.09 μM[2]. CAN508 (40 μM; 72 hours) increases apoptosis in all three esophageal adenocarcinoma cells[2]. Apoptosis Analysis[1] Cell Line: SKGT4, OE33 and FLO-1 cells Concentration: 40 μM Incubation Time: 72 hours Result: Increased apoptosis by 2 fold in all three esophageal adenocarcinoma cells compared to untreated controls. |
| In Vivo | CAN508 (60 mg/kg; i.p.; daily for 10 days) has antitumor effects in esophageal adenocarcinoma xenografts[1]. Animal Model: 4 weeks-old female nude mice (esophageal adenocarcinoma xenografts)[1] Dosage: 60 mg/kg Administration: I.p.; daily for 10 days Result: Caused reduction of tumor growth starting from post-treatment day three with 50.83% reduction. |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 638.3±55.0 °C at 760 mmHg |
| Molecular Formula | C9H10N6O |
| Molecular Weight | 218.215 |
| Flash Point | 339.8±31.5 °C |
| Exact Mass | 218.091614 |
| PSA | 125.67000 |
| LogP | 0.40 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.803 |
| 3tn8 |
| 2clx |
| Cdk9 Inhibitor II |
| Phenol, 4-[(E)-2-(3,5-diamino-1H-pyrazol-4-yl)diazenyl]- |
| 4-[(E)-(3,5-Diamino-1H-pyrazol-4-yl)diazenyl]phenol |
| F18 |
| CAN-508 |