Dronedarone hydrochloride
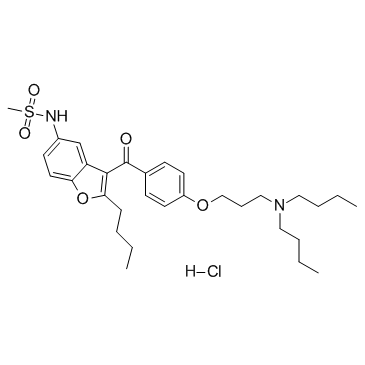
Dronedarone hydrochloride structure
|
Common Name | Dronedarone hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 141625-93-6 | Molecular Weight | 593.217 | |
| Density | N/A | Boiling Point | 683.9ºC at 760mmHg | |
| Molecular Formula | C31H45ClN2O5S | Melting Point | NA (low-melting) | |
| MSDS | Chinese USA | Flash Point | 367.4ºC | |
Use of Dronedarone hydrochlorideDronedarone hydrochloride is a non-iodinated amiodarone derivative that inhibits Na+, K+ and Ca2+ currents. |
| Name | Dronedarone Hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Dronedarone hydrochloride is a non-iodinated amiodarone derivative that inhibits Na+, K+ and Ca2+ currents. |
|---|---|
| Related Catalog | |
| In Vitro | Dronedarone (SR-33589) is a multichannel blocker for atrial fibrillation . It is a potent inhibitor of the acetylcholine-activated K+ current from atrial and sinoatrial nodal tissue, and inhibits the rapid delayed rectifier more potently than slow and inward rectifier K+ currents and inhibits L-type calcium current. Under whole-cell patch clamp, it blocks IKr (IC50=3 μM) and ICa-L (IC50=0.18 μM). The effects on ICa-L are use- and frequency-dependent. Dronedarone inhibits current carried by human ether-a-go-go gene (HERG)-expressing oocytes (analagous to IKr) with an IC50 of 9 μM[1]. In guinea pig ventricular myocytes, dronedarone exhibits a state dependent inhibition of the fast Na+ channel current with an IC50 of 0.7±0.1 μM, when the holding potential is −80 mV[2]. |
| In Vivo | Dronedarone (Hydrochloride) reduces significantly the incidence of ventricular fibrillation (VF) from 80 to 30% (p < 0.05) at 3 mg/kg i.v. and eliminated VF and mortality at 10 mg/kg i.v. [3]. Dronedarone inhibited carotid artery thrombus formation in vivo. Thrombin- and collagen-induced platelet aggregation is impaired indronedarone-treated mice (P\0.05), and expression ofplasminogen activator inhibitor-1 (PAI1), an inhibitor of the fibrinolytic system, is reduced in the arterial wall[4]. |
| Animal Admin | Rats: Rats are anesthetized, artificially ventilated, and the thorax opened by a left thoracotomy. Ischemia is induced by left coronary artery ligation, and reperfusion is achieved (after a 5-min period of ischemia) in a separate group of rats by removing the ligature. Agents are given intravenously 5 min before occlusion or orally 4 h before study[3]. Mice: Twelve-week-old C57Bl/6 mice are divided into two groups: dronedarone (200 mg/kg body weight with a once daily oral gavage for 14 days) or control (1.4 % methylcellulose). Twenty-four hours after the last application, mice are anesthetized by intraperitoneal injection of 87 mg/kg sodium pentobarbital. Rose bengal is diluted to 12 mg/mL in phosphate-buffered saline and then injected into the tail vein at a concentration of 63 mg/kg[4]. |
| References |
| Boiling Point | 683.9ºC at 760mmHg |
|---|---|
| Melting Point | NA (low-melting) |
| Molecular Formula | C31H45ClN2O5S |
| Molecular Weight | 593.217 |
| Flash Point | 367.4ºC |
| Exact Mass | 592.273743 |
| PSA | 97.23000 |
| LogP | 9.00480 |
| Appearance of Characters | white to off-white |
| Vapour Pressure | 1.47E-18mmHg at 25°C |
| Storage condition | Refrigerator |
| Water Solubility | DMSO: soluble15mg/mL, clear |
| RIDADR | UN 3077 9 / PGIII |
|---|
|
Use of In Vitro Morphogenesis of Mouse Embryoid Bodies to Assess Developmental Toxicity of Therapeutic Drugs Contraindicated in Pregnancy.
Toxicol. Sci. 149 , 15-30, (2016) In utero exposure to certain chemicals can impair embryo development, causing embryonic death, growth retardation, or severe birth defects. Establishment of effective in vitro tests is crucial for ide... |
|
|
Inactivation of Human Cytochrome P450 3A4 and 3A5 by Dronedarone and N-Desbutyl Dronedarone.
Mol. Pharmacol. 89 , 1-13, (2015) Dronedarone is an antiarrhythmic agent approved in 2009 for the treatment of atrial fibrillation. An in-house preliminary study demonstrated that dronedarone inhibits cytochrome P450 (CYP) 3A4 and 3A5... |
| n-(2-butyl-3-(4-(3-(dibutylamino)propoxy)benzoyl)-5-benzofuranyl)methanesulfonamide monohydrochloride |
| N-(2-Butyl-3-{4-[3-(dibutylamino)propoxy]benzoyl}-1-benzofuran-5-yl)methanesulfonamide hydrochloride (1:1) |
| Methanesulfonamide, N-[2-butyl-3-[4-[3-(dibutylamino)propoxy]benzoyl]-5-benzofuranyl]-, hydrochloride (1:1) |
| Multaq Hydrochloride. |
| Methanesulfonamide, N-(2-butyl-3-(4-(3-(dibutylamino)propoxy)benzoyl)-5-benzofuranyl)-, monohydrochloride |
| Multaq |
| Dronedarone (Hydrochloride) |
| Dronedarone hydrochloride |
| Dronedarone HCl |

