Amino-Tri-(carboxyethoxymethyl)-methane hydrochloride
Modify Date: 2024-04-02 21:44:29
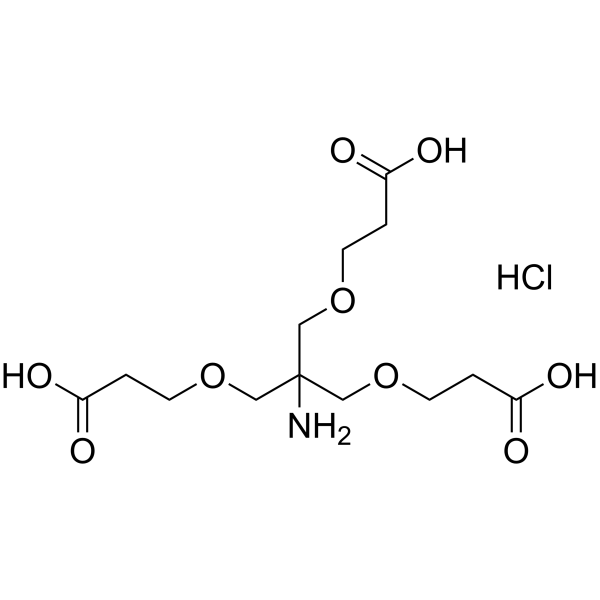
Amino-Tri-(carboxyethoxymethyl)-methane hydrochloride structure
|
Common Name | Amino-Tri-(carboxyethoxymethyl)-methane hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 1416771-72-6 | Molecular Weight | 373.78 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C13H24ClNO9 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Amino-Tri-(carboxyethoxymethyl)-methane hydrochlorideAmino-Tri-(carboxyethoxymethyl)-methane hydrochloride is a cleavable PEG ADC linker used in the synthesis of antibody-drug conjugates (ADCs). Amino-Tri-(carboxyethoxymethyl)-methan hydrochloride is also a PEG-based PROTAC linker that can be used in the synthesis of PROTACs[1][2]. |
| Name | Amino-Tri-(carboxyethoxymethyl)-methane hydrochloride |
|---|
| Description | Amino-Tri-(carboxyethoxymethyl)-methane hydrochloride is a cleavable PEG ADC linker used in the synthesis of antibody-drug conjugates (ADCs). Amino-Tri-(carboxyethoxymethyl)-methan hydrochloride is also a PEG-based PROTAC linker that can be used in the synthesis of PROTACs[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | ADCs are comprised of an antibody to which is attached an ADC cytotoxin through an ADC linker[1]. PROTACs contain two different ligands connected by a linker; one is a ligand for an E3 ubiquitin ligase and the other is for the target protein. PROTACs exploit the intracellular ubiquitin-proteasome system to selectively degrade target proteins[2]. |
| References |
[1]. Markus Ribbert, et al. Self coupling recombinant antibody fusion proteins. WO2009013359A2. |
| Molecular Formula | C13H24ClNO9 |
|---|---|
| Molecular Weight | 373.78 |