Clobenpropit dihydrobromide
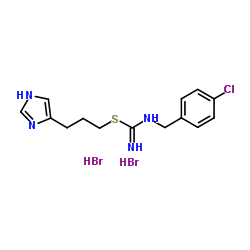
Clobenpropit dihydrobromide structure
|
Common Name | Clobenpropit dihydrobromide | ||
|---|---|---|---|---|
| CAS Number | 145231-35-2 | Molecular Weight | 470.654 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C14H19Br2ClN4S | Melting Point | 205 °C | |
| MSDS | N/A | Flash Point | N/A | |
Use of Clobenpropit dihydrobromideClobenpropit dihydrobromide is a potent histamine H3R antagonist/inverse agonist with a pEC50 of 8.07 for histamine H3LR[1]. Clobenpropit dihydrobromide acts as partial agonist at histamine H4 receptors (Ki 13 nM). Clobenpropit dihydrobromide also binds to serotonin 5-HT3 receptors (Ki 7.4 nM) and α2A/α2C adrenoceptors (Ki 17.4/7.8 nM)[2]. Clobenpropit dihydrobromide increases apoptosis[3]. |
| Name | clobenpropit dihydrobromide |
|---|---|
| Synonym | More Synonyms |
| Description | Clobenpropit dihydrobromide is a potent histamine H3R antagonist/inverse agonist with a pEC50 of 8.07 for histamine H3LR[1]. Clobenpropit dihydrobromide acts as partial agonist at histamine H4 receptors (Ki 13 nM). Clobenpropit dihydrobromide also binds to serotonin 5-HT3 receptors (Ki 7.4 nM) and α2A/α2C adrenoceptors (Ki 17.4/7.8 nM)[2]. Clobenpropit dihydrobromide increases apoptosis[3]. |
|---|---|
| Related Catalog | |
| Target |
Human H3LR:9.44 (pKi) Rat H3LR:9.75 (pKi) H4 receptor:13 nM (Ki) H2 Receptor:5.6 (pKi) |
| In Vitro | Clobenpropit binds to human H3LR and rat H3LR with pKis of 9.44±0.04 and 9.75±0.01. Clobenpropit exhibits low affinity for histamine H1R or H2R (pKis of 5.2 and 5.6, respectively)[1]. Clobenpropit inhibits [3H]-dopamine transport by SH-SY5Y cells in a concentration dependent manner with maximum inhibition 82.7±2.8 % and IC50 490 nM (pIC50 6.31±0.11)[2]. Clobenpropit is a subunit-selective noncompetitive antagonist at recombinant NMDA receptors (IC50 1 μM for the NR1/NR2B receptor)[2]. Clobenpropit (50 μM) and Gemcitabine (5 μM) combination therapy significantly increases apoptosis of Panc-1, MiaPCa-2 and AsPC-1 compared with control[3]. Apoptosis Analysis[3] Cell Line: Pancreatic cancer cells (Panc-1, MiaPaCa-2 and AsPC-1) Concentration: 50 μM Incubation Time: Result: Enhanced apoptotic cell death in combination of Gemcitabine (5 μM). |
| In Vivo | The combination treatment of Clobenpropit (every other day intraperitoneal injection at 20 μM per kilogram for 40 d) and Gemcitabine (twice-a-week intraperitoneal injection at 125 mg/kg for 40 d) shows significant tumor growth inhibition[3]. Animal Model: Five-week-old male BALB/c nude mice with Panc-1 xenograft[3] Dosage: 20 μM per kilogram Administration: Intraperitoneal injection; every other day for 40 days. Gemcitabine (twice-a-week intraperitoneal injection at 125 mg/kg for 40 d) Result: The combination treatment showed significant tumor growth inhibition compared with other treatment groups (control 501±92 mg, Gemcitabine 294±46 mg, Clobenpropit 444±167 mg, and combination 154±54 mg). |
| References |
| Melting Point | 205 °C |
|---|---|
| Molecular Formula | C14H19Br2ClN4S |
| Molecular Weight | 470.654 |
| Exact Mass | 467.938568 |
| PSA | 89.86000 |
| LogP | 5.86030 |
|
~% 
Clobenpropit di... CAS#:145231-35-2 |
| Literature: Van der Goot; Schepers; Sterk; Timmerman European Journal of Medicinal Chemistry, 1992 , vol. 27, # 5 p. 511 - 517 |
|
~% 
Clobenpropit di... CAS#:145231-35-2 |
| Literature: Van der Goot; Schepers; Sterk; Timmerman European Journal of Medicinal Chemistry, 1992 , vol. 27, # 5 p. 511 - 517 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| 1H-Imidazole-5-propanol |
| 3-(1H-Imidazol-4-yl)propyl N-(4-chlorobenzyl)carbamimidothioate dihydrobromide |
| Carbamimidothioic acid, N-[(4-chlorophenyl)methyl]-, 3-(1H-imidazol-4-yl)propyl ester, hydrobromide (1:2) |
| Clobenpropit dihydrobromide |
| Clobenpropit (hydrobromide) |