Sch-42495 racemate
Modify Date: 2024-01-02 12:37:59
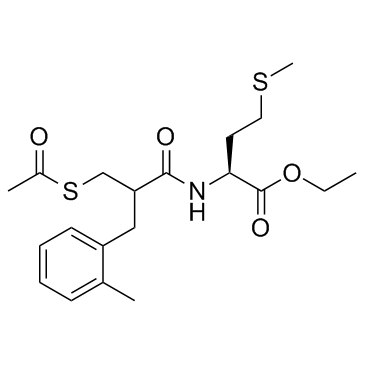
Sch-42495 racemate structure
|
Common Name | Sch-42495 racemate | ||
|---|---|---|---|---|
| CAS Number | 145841-10-7 | Molecular Weight | 411.579 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 597.3±50.0 °C at 760 mmHg | |
| Molecular Formula | C20H29NO4S2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 315.0±30.1 °C | |
Use of Sch-42495 racemateSch-42495 racemate is the racemate of Sch-42495. Sch-42495 is a novel neutral metalloendopeptidase (NEP) inhibitor. Sch-42495 is the orally active ethylester prodrug of SCH 42354. |
| Name | Sch-42495 racemate |
|---|---|
| Synonym | More Synonyms |
| Description | Sch-42495 racemate is the racemate of Sch-42495. Sch-42495 is a novel neutral metalloendopeptidase (NEP) inhibitor. Sch-42495 is the orally active ethylester prodrug of SCH 42354. |
|---|---|
| Related Catalog | |
| Target |
neutral metalloendopeptidase (NEP)[1] |
| In Vitro | SCH 42354 selectively inhibits hydrolysis of leu-enkephalin and ANF (IC50 of 8.3 and 10.0 nM, respectively) in vitro[1]. |
| In Vivo | Plasma levels of exogenous atrial natriuretic factor (ANF) are augmented and ANF clearance from plasma is delayed by oral Sch-42495 (3 to 30 mg/kg) in normotensive rats. Plasma ANF levels in volume expanded rats are higher in Sch-42495-treated rats. Diuretic and natriuretic effects of ANF are increased in rats treated with Sch-42495. In Dahl-S hypertensive rats, Sch-42495 (1 to 10 mg/kg orally) produces falls in blood pressure of a magnitude similar to that observed in DOCA-Na hypertensive rats. Significant hypotensive activity is observed 18 h after a single 10 mg/kg oral dose in Dahl-S hypertensive rats. In DOCA-Na hypertensive rats, a single dose of Sch-42495 significantly decreases cardiac output and does not lower systemic vascular resistance, a profile similar to that of ANF[1]. Spontaneously hypertensive rats (SHR) aged 9 to 10 weeks are injected with either Streptozotocin (45 mg/kg) or citrate buffer and randomized to receive either Captopril (25 mg/kg BID), Sch-42495 (30 mg/kg BID), S21402 (25 or 50 mg/kg BID), or vehicle by gavage for 4 weeks. A group of diabetic SHR is also allocated to receive the combination of Sch-42495 (30 mg/kg BID) and Captopril (25 mg/kg BID). The degree of renal NEP inhibition is determined by autoradiography, and plasma renin activity (PRA) is determined by radioimmunoassay. In nondiabetic SHR, S21402 and Captopril are equally effective. Relative heart weight decreases in parallel to the changes in blood pressure. Renal NEP is clearly inhibited (70% to 92%; P<0.001) by both Sch-42495 and S21402[2]. |
| Animal Admin | Rats[2] Streptozotocin-injected and citrate buffer–injected SHR are randomly allocated to receive by gavage Captopril Captopril (25 mg/kg BID in distilled water), Sch-42495 (30 mg/kg BID in 5% arabic gum), S21402 at 2 different doses (25 mg/kg BID or 50 mg/kg BID in 5% arabic gum), or vehicle for 4 weeks starting on the day of Streptozotocin or buffer injection. The choice of the 2 doses of S21402 is based on previous findings suggesting that sufficient renal NEP inhibition, comparable to 30 mg/kg of Sch-42495, may be obtained with 25 mg/kg of S21402, while 50 mg/kg of S21402 is needed to provide equipotent angiotensin I pressor response inhibitory efficacy compared with 25 mg/kg of Captopril. A group of diabetic SHR is also allocated to receive the combination of Sch-42495 (30 mg/kg BID) and Captopril (25 mg/kg BID) by gavage. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 597.3±50.0 °C at 760 mmHg |
| Molecular Formula | C20H29NO4S2 |
| Molecular Weight | 411.579 |
| Flash Point | 315.0±30.1 °C |
| Exact Mass | 411.153809 |
| LogP | 4.07 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.548 |
| L-Methionine, N-[3-(acetylthio)-2-[(2-methylphenyl)methyl]-1-oxopropyl]-, ethyl ester |
| Ethyl N-[3-(acetylsulfanyl)-2-(2-methylbenzyl)propanoyl]-L-methioninate |