D-allo-Isoleucine
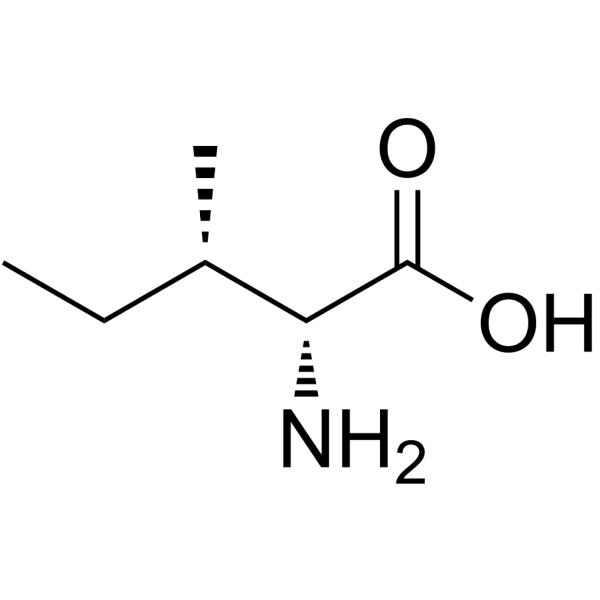
D-allo-Isoleucine structure
|
Common Name | D-allo-Isoleucine | ||
|---|---|---|---|---|
| CAS Number | 1509-35-9 | Molecular Weight | 131.173 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 225.8±23.0 °C at 760 mmHg | |
| Molecular Formula | C6H13NO2 | Melting Point | 291 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 90.3±22.6 °C | |
Use of D-allo-IsoleucineD-Alloisoleucine is an isoleucine derivative[1]. |
| Name | D-alloisoleucine |
|---|---|
| Synonym | More Synonyms |
| Description | D-Alloisoleucine is an isoleucine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 225.8±23.0 °C at 760 mmHg |
| Melting Point | 291 °C (dec.)(lit.) |
| Molecular Formula | C6H13NO2 |
| Molecular Weight | 131.173 |
| Flash Point | 90.3±22.6 °C |
| Exact Mass | 131.094635 |
| PSA | 63.32000 |
| LogP | 0.73 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.463 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | BA2930000 |
| HS Code | 2922499990 |
| Precursor 0 | |
|---|---|
| DownStream 2 | |
| HS Code | 2922499990 |
|---|---|
| Summary | HS:2922499990 other amino-acids, other than those containing more than one kind of oxygen function, and their esters; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward) MFN tariff:6.5% General tariff:30.0% |
|
Synthesis of the amino acid conjugates of epi-jasmonic acid.
Amino Acids 42(5) , 1955-66, (2011) The TES ether of the C6-hydroxy derivative of naturally occurring epi-jasmonic acid (epi-JA) was designed as epimerization-free equivalent of epi-JA. The TES ether was synthesized from (1R,4S)-4-hydro... |
|
|
Antimicrobial peptides from amphibian skin: an overview.
Ciba Found. Symp. 186 , 77-85, (1994) Over the past three decades, numerous peptides have been isolated from amphibian skin secretions. Many of these peptides were shown to be homologous to hormones and neurotransmitters of mammals. In re... |
|
|
Total synthesis of the bicyclic depsipeptide HDAC inhibitors spiruchostatins A and B, 5''-epi-spiruchostatin B, FK228 (FR901228) and preliminary evaluation of their biological activity.
Chemistry 15 , 11174-11186, (2009) The bicyclic depsipeptide histone deacetylase (HDAC) inhibitors spiruchostatins A and B, 5''-epi-spiruchostatin B and FK228 were efficiently synthesized in a convergent and unified manner. The synthet... |
| D-alloisoleucine zwitterion |
| d-alloisoleucin |
| (2R,3S)-2-Amino-3-methylpentanoic acid |
| MFCD00066445 |
| threo-d-isoleucine |
| EINECS 216-143-9 |
| D-ALLO-A-AMINO-B-METHYLVALERIC ACID |
| Alloisoleucine |
| D-allo-Isoleucine |
| Alloisoleucine, D- |
| D-allo-Ile-OH |
| H-D-allo-lle-OH |
| allo-Ile |
| D-ALLO-2-AMINO-3-METHYLPENTANOIC ACID |
| D-Alloisoleucine |
| allo-D-isoleucine |
| H-D-allo-Ile-OH |
 CAS#:1460-34-0
CAS#:1460-34-0 CAS#:6893-26-1
CAS#:6893-26-1
