Ifetroban sodium
Modify Date: 2024-01-29 21:35:26
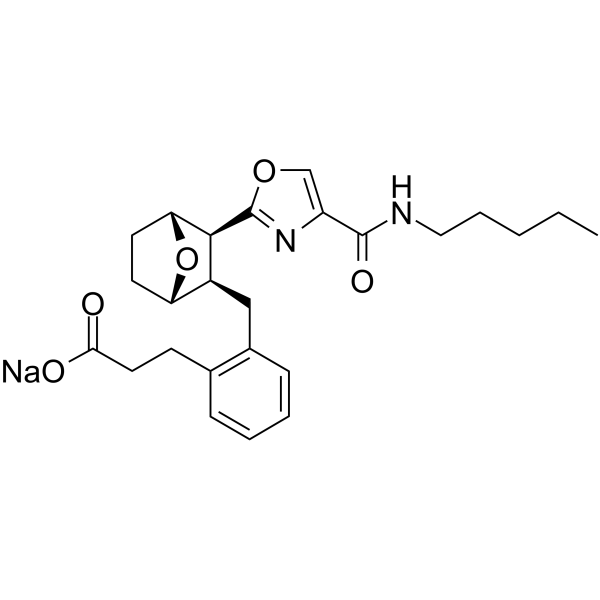
Ifetroban sodium structure
|
Common Name | Ifetroban sodium | ||
|---|---|---|---|---|
| CAS Number | 156715-37-6 | Molecular Weight | 462.51400 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C25H31N2NaO5 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Ifetroban sodiumIfetroban (BMS-180291) sodium is an orally active antagonist of thromboxane A2 (TXA2) or prostaglandin H2 (PGH2) receptor. Ifetroban sodium shows antiplatelet activity, and inhibits tumor cell migration without affecting cell proliferation. Ifetroban sodium can be used for myocardial ischemia, hypertension, stroke, thrombosis, cardiomyopathy research[1][2][3][4]. |
| Name | ifetroban sodium |
|---|---|
| Synonym | More Synonyms |
| Description | Ifetroban (BMS-180291) sodium is an orally active antagonist of thromboxane A2 (TXA2) or prostaglandin H2 (PGH2) receptor. Ifetroban sodium shows antiplatelet activity, and inhibits tumor cell migration without affecting cell proliferation. Ifetroban sodium can be used for myocardial ischemia, hypertension, stroke, thrombosis, cardiomyopathy research[1][2][3][4]. |
|---|---|
| Related Catalog | |
| Target |
Thromboxane A2 receptor; Prostaglandin H2 receptor[4] |
| In Vitro | Ifetroban sodium (CPI211) (100 nM; 48 h) results Tpr inhibition and potently blocks spontaneous metastasis from primary tumors, without affecting tumor cell proliferation, motility, or tumor growth in 4T1 cells (mouse mammary cancer)[2]. Ifetroban sodium (100 nM; 6 h) strongly inhibits PKC substrate phosphorylation, and blocks agonist (U46619, HY-108566)-induced TPr diminution in human umbilical vein endothelial cells (HUVECs)[2]. Western Blot Analysis[2] Cell Line: Mouse pulmonary microvascular endothelial cells (MPMECs) and human umbilical vein endothelial cells (HUVECs) Concentration: 100 nM Incubation Time: 6 hours Result: Decreased the level of TPr protein and inhibited PKC substrate phosphorylation. Immunofluorescence[2] Cell Line: Mouse pulmonary microvascular endothelial cells (MPMECs) and human umbilical vein endothelial cells (HUVECs) Concentration: 100 nM Incubation Time: 6 hours Result: Showed transendothelial migration of GFP+ 4T1 and MDA-MB-231 across mouse MPMECs and human HUVECs. |
| In Vivo | Ifetroban sodium (50 mg/kg/d; p.o.; 2 d prior to, through 28 d after tumor injection) decreases hematogenous metastasis of multiple cancer types without in mice model[2]. Ifetroban sodium (50 mg/kg/d; p.o.; 12 d) does not affect primary tumor growth but decreases tumor vessels in mice with 4T1 (mouse mammary cancer)[2]. Ifetroban sodium (BMS 180,291; 1 and 3 mg/kg, p.o.) inhibits aggregation and antagonizes TP-receptor in monekys. Ifetroban sodium (3 mg/kg, i.v.) causes only marginal and transient hemodynamic effects in anesthetized African green monkeys[3]. Animal Model: Athymic (nu/nu) Balb/C female mice injected with tumor cells: 4T1 (mouse mammary cancer), MDA-MB-231 (human breast cancer), MiaPaCa2 (human pancreatic cancer), and A549 (human lung cancer) model[2] Dosage: 50 mg/kg; via 25 μL vehicle (4% sucrose in sterile water) Administration: Oral gavage; pretreated before 2 days and treated 28 days later Result: Decreased the percentage of mice harboring MDA-MB-231 lung metastases from 90% to 20%, and mice with A549 lung metastases from 60% to 10%. |
| References |
| Molecular Formula | C25H31N2NaO5 |
|---|---|
| Molecular Weight | 462.51400 |
| Exact Mass | 462.21300 |
| PSA | 104.49000 |
| LogP | 3.17170 |
| [1S-(1α,2α,3α,4α)]-2-[[3-[4-[(Pentylamino)carbonyl]-2-oxazolyl]-7-oxabicyclo[2.2.1]hept-2-yl]methyl]benzenepropanoic acid,monosodium salt |
| IFETROBAN SODIUM |
| [1S-(1α,2α,3α,4α)]-2-[[3-[4-[(Pentylamino)carbonyl]-2-oxazolyl]-7-oxabicyclo[2.2.1]hept-2-yl]methyl]-benzenepropanoic acid,monosodium salt |
| [1S-(1α,2α,3α,4α)]-2-[[3-[4-[(pentylamino)carbonyl]-2-oxazolyl]-7-oxabicyclo[2.2.1]hept-2-yl]methyl]-benzenepropanoic acid monosodium salt |