Indinavir Sulfate
Modify Date: 2024-01-02 06:24:57
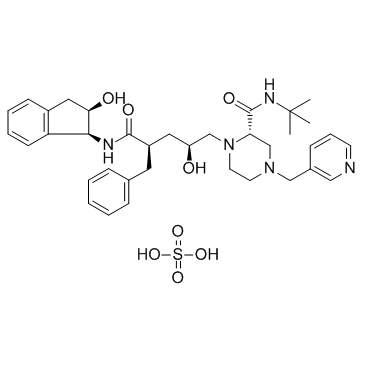
Indinavir Sulfate structure
|
Common Name | Indinavir Sulfate | ||
|---|---|---|---|---|
| CAS Number | 157810-81-6 | Molecular Weight | 711.868 | |
| Density | N/A | Boiling Point | 877.9ºC at 760 mmHg | |
| Molecular Formula | C36H49N5O8S | Melting Point | 150-153ºC | |
| MSDS | N/A | Flash Point | 484.7ºC | |
Use of Indinavir SulfateIndinavir sulfate(MK-639 sulfate; L735524 sulfate ) is a potent and specific HIV protease inhibitor that appears to have good oral bioavailability.Target: HIV ProteaseIndinavir(MK-639) is a protease inhibitor used as a component of highly active antiretroviral therapy (HAART) to treat HIV infection and AIDS.MK-639 appears to have significant dose-related antiviral activity and is well tolerated [1]. Inhibition constants (K(i)) of the antiviral drug indinavir for the reaction catalyzed by the mutant enzymes were about threefold and 50-fold higher for PR(L24I) and PR(I50V), respectively, relative to PR and PR(G73S). The dimer dissociation constant (K(d)) was estimated to be approximately 20 nM for both PR(L24I) and PR(I50V), and below 5 nM for PR(G73S) and PR. Crystal structures of the mutants PR(L24I), PR(I50V) and PR(G73S) were determined in complexes with indinavir, or the p2/NC substrate analog at resolutions of 1.10-1.50 Angstrom [2]. |
| Name | Indinavir sulfate |
|---|---|
| Synonym | More Synonyms |
| Description | Indinavir sulfate(MK-639 sulfate; L735524 sulfate ) is a potent and specific HIV protease inhibitor that appears to have good oral bioavailability.Target: HIV ProteaseIndinavir(MK-639) is a protease inhibitor used as a component of highly active antiretroviral therapy (HAART) to treat HIV infection and AIDS.MK-639 appears to have significant dose-related antiviral activity and is well tolerated [1]. Inhibition constants (K(i)) of the antiviral drug indinavir for the reaction catalyzed by the mutant enzymes were about threefold and 50-fold higher for PR(L24I) and PR(I50V), respectively, relative to PR and PR(G73S). The dimer dissociation constant (K(d)) was estimated to be approximately 20 nM for both PR(L24I) and PR(I50V), and below 5 nM for PR(G73S) and PR. Crystal structures of the mutants PR(L24I), PR(I50V) and PR(G73S) were determined in complexes with indinavir, or the p2/NC substrate analog at resolutions of 1.10-1.50 Angstrom [2]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 877.9ºC at 760 mmHg |
|---|---|
| Melting Point | 150-153ºC |
| Molecular Formula | C36H49N5O8S |
| Molecular Weight | 711.868 |
| Flash Point | 484.7ºC |
| Exact Mass | 711.330200 |
| PSA | 201.01000 |
| LogP | 3.95250 |
| Vapour Pressure | 5.56E-33mmHg at 25°C |
| Storage condition | -20°C Freezer |
| Water Solubility | Freely soluble in water, soluble in methanol, practically insoluble in heptane. |
| Safety Phrases | S36/37 |
|---|---|
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| MK-639,Crixivan |
| Indinivar sulphate |
| Indinavir Sulfate |
| INDINAVIR SULPHATE |
| Indinavir (sulfate) |
| indinavirsulfate(subjecttopatentfree) |
| (2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-5-{[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]amino}-5-oxopentyl]-N-tert-butyl-4-(pyridin-3-ylmethyl)piperazine-2-carboxamide sulfate (salt) |
| (2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-5-{[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]amino}-5-oxopentyl]-N-tert-butyl-4-(pyridin-3-ylmethyl)piperazine-2-carboxamide sulfate (salt) (non-preferred name) |
| (2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-5-{[(1S,2R)-2-hydroxy-2,3-dihydro-1H-indén-1-yl]amino}-5-oxopentyl]-N-(1,1-diméthyléthyl)-4-(pyridin-3-ylméthyl)pipérazine-2-carboxamide sulfate (salt) |
| Crixivan |
| MK-639 |
| INDIGOWOADROOTEXTRACT |
| IDV |
| (2S)-1-[(2S,4R)-4-Benzyl-2-hydroxy-5-{[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]amino}-5-oxopentyl]-N-tert-butyl-4-(pyridin-3-ylmethyl)piperazine-2-carboxamide sulfate (1:1) (non-preferred name) |
| (2S)-1-[(2S,4R)-4-Benzyl-2-hydroxy-5-{[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]amino}-5-oxopentyl]-N-(2-methyl-2-propanyl)-4-(3-pyridinylmethyl)-2-piperazinecarboxamide sulfate (1:1) (non-preferred name) |
| (2S)-1-[(2S,4R)-4-Benzyl-2-hydroxy-5-{[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]amino}-5-oxopentyl]-N-tert-butyl-4-(pyridin-3-ylmethyl)piperazin-2-carboxamidsulfat(salt) |