beta-Amyrin acetate
Modify Date: 2024-01-09 21:04:03
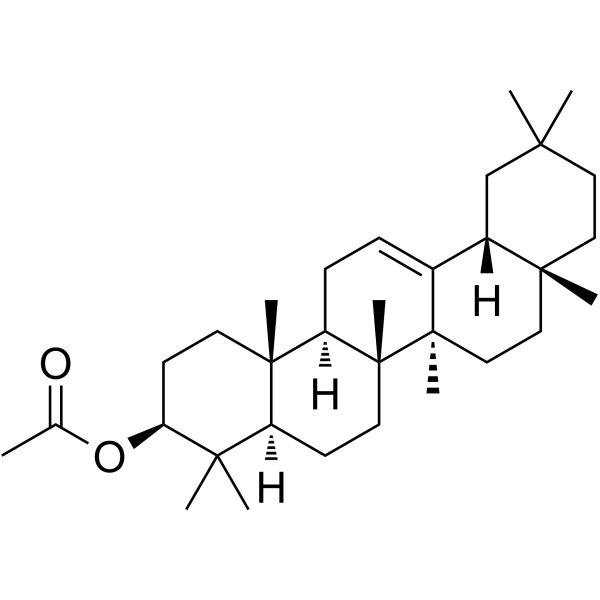
beta-Amyrin acetate structure
|
Common Name | beta-Amyrin acetate | ||
|---|---|---|---|---|
| CAS Number | 1616-93-9 | Molecular Weight | 468.754 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 505.1±49.0 °C at 760 mmHg | |
| Molecular Formula | C32H52O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 256.2±17.4 °C | |
Use of beta-Amyrin acetateβ-Amyrin acetate is a triterpenoid with potent anti-inflammatory, antifungal, anti-diabetic, anti-hyperlipidemic activities. β-Amyrin acetate can inhibit HMG-CoA reductase activity by locating in the hydrophobic binding cleft of HMG CoA reductase[1][2][3][4]. |
| Name | (4,4,6a,6b,8a,11,11,14b-octamethyl-1,2,3,4a,5,6,7,8,9,10,12,12a,14,14a-tetradecahydropicen-3-yl) acetate |
|---|---|
| Synonym | More Synonyms |
| Description | β-Amyrin acetate is a triterpenoid with potent anti-inflammatory, antifungal, anti-diabetic, anti-hyperlipidemic activities. β-Amyrin acetate can inhibit HMG-CoA reductase activity by locating in the hydrophobic binding cleft of HMG CoA reductase[1][2][3][4]. |
|---|---|
| Related Catalog | |
| In Vitro | β-Amyrin acetate (50 μg/mL) inhibits heat-induced hemolysis and hypotonicity-induced hemolysis of human erythrocytes[1]. β-Amyrin acetate (5-100 μM) has HMG-CoA reductase inhibitory activity by locating in the hydrophobic binding cleft lined with residues Leu562, Gly560, Ala564, Gly569, Ser852, Leu853, Leu857, Met854, Ala856, Ser852 and Ala855 of human HMG CoA reductase[2]. β-Amyrin acetate (7.8-1000 μg/mL, 48 h) inhibits all of the Candida fungal species tested with MIC values ranging from 30 to 250 μg/mL)[4]. |
| In Vivo | β-Amyrin acetate (applied on the anterior surface of the right ear, 100 μg/ear, a single dose) significantly inhibits xylene-induced ear edema in mice[1]. β-Amyrin acetate (intraperitoneal injection, 4 mg/100 g, daily for 6 consecutive days) shows significant anti-inflammatory activities (43.6%) in adult albino rats[3]. β-Amyrin acetate (subcutaneous injection, 4 mg/100 g, daily for 10 days ) increases the ATP-phosphohydrolase activity in liver homogenates both in normal and arthritic rats[3]. Animal Model: Xylene-induced mouse ear topical edema model[1] Dosage: 50 and 100 μg/ear (5 μL) Administration: Applied on the anterior surface of the right ear Result: Inhibited Xylene-induced ear edema in mice Animal Model: Adult albino rats[3] Dosage: 4 mg/100g, daily for 6 consecutive days Administration: Intraperitoneal injection Result: Showed significant anti-inflammatory activities with mean average weight of granulation tissue of 9.2 mg after 6 days. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 505.1±49.0 °C at 760 mmHg |
| Molecular Formula | C32H52O2 |
| Molecular Weight | 468.754 |
| Flash Point | 256.2±17.4 °C |
| Exact Mass | 468.396729 |
| PSA | 26.30000 |
| LogP | 11.95 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.529 |
| Hazard Codes | Xi |
|---|
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| Olean-12-en-3β-ol, acetate |
| (3β)-Olean-12-en-3-ylacetat |
| Acétate de (3β)-olean-12-én-3-yle |
| UNII:AM1H7102OC |
| (3S,4aR,6aR,6bS,8aR,12aR,14aR,14bR)-4,4,6a,6b,8a,11,11,14b-Octamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydro-3-picenyl acetate |
| Olean-12-en-3-ol, acetate, (3β)- |
| AMYRIN,B |
| Olean-12-en-3β-ol, acetate (8CI) |
| 3-β-acetoxyolean-12-ene |
| O-Acetyl-β-amyrin |
| (3β)-Olean-12-en-3-yl acetate |
| β-Amyrin acetate |
| (3S,4aR,6aR,6bS,8aR,12aR,14aR,14bR)-4,4,6a,6b,8a,11,11,14b-Octamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydro-3-picenylacetat |
| Olean-12-en-3-yl acetate |
| Acétate de (3S,4aR,6aR,6bS,8aR,12aR,14aR,14bR)-4,4,6a,6b,8a,11,11,14b-octaméthyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydro-3-picényle |
 CAS#:559-70-6
CAS#:559-70-6
